Translate this page into:
Guideline-directed medical therapy use in unfunded patients with heart failure with reduced ejection fraction within an academic health system
*Corresponding author: Jillian Kelsey Contreras, PharmD, BCACP Department of Pharmacotherapy and Pharmacy Services, University Health, San Antonio, Texas, United States. jillian.contreras@utexas.edu
-
Received: ,
Accepted: ,
How to cite this article: Contreras JK, Hernandez W, Frei CR, et al. Guideline-directed medical therapy use in unfunded patients with heart failure with reduced ejection fraction within an academic health system. Am J Pharmacother Pharm Sci 2025:008.
Abstract
Objectives
Heart failure (HF) remains a major cause of hospitalizations and mortality globally, despite advances in therapeutics. Quadruple therapy is the foundation for guideline-directed medical therapy (GDMT) of HF with reduced ejection fraction (HFrEF). Despite proven benefits, proper GDMT in everyday practice is underutilized. The purpose of this study is to evaluate outpatient prescribing patterns of GDMT in unfunded HFrEF patients and its impact on clinical outcomes within a large county health system.
Materials and Methods
A retrospective chart review was conducted for unfunded adult outpatients with a visit diagnosis of HFrEF based on an echocardiogram. Information collected for each study subject included rates of prescribed GDMT, target doses achieved, adherence, pharmacist involvement in dose titration, and hospitalizations/mortality.
Results
Of the 3219 patients with a chart diagnosis of HFrEF, 232 patients met the inclusion criteria. Triple therapy was prescribed in 60.3% of patients, while 36.2% were prescribed quadruple therapy. Among the study population, 87.1% were on guideline-directed beta-blocker (BB), 48.3% on angiotensin-converting enzyme inhibitors (ACEI), or angiotensin receptor blockers (ARB), 31% on angiotensin receptor/neprilysin inhibitor (ARNI), 72.4% on a mineralocorticoid receptor antagonist (MRA), and 52.2% were on a sodium-glucose cotransporter 2 inhibitor (SGLT2i). Prescribing rates of target doses of ACEI/ARB, ARNI, BB, MRAs, and SGLT2i were 29.5%, 13.9%, 23.2%, 81.5%, and 90.1% respectively. GDMT titration was performed by pharmacists on 12.9% of patients.
Conclusion
Significant underutilization of GDMT and optimal dose titration remain within a large health system. Clinical pharmacists within our institution were intermittently involved in optimizing GDMT within the patient population. This study suggests there are opportunities for pharmacists to assist with GDMT optimization.
Keywords
Dose titration
Guideline-directed medical therapy
Heart failure
Pharmacist intervention
Unfunded
INTRODUCTION
Heart failure (HF) is a major public health issue, affecting over 6 million adults in the United States. Every year there are more than 1 million HF hospitalizations. Among patients hospitalized for HF, approximately 20% of them are readmitted within 30 days.[1] Goals for these patients include a reduction in hospitalizations and mortality by optimizing guideline-directed medical therapy (GDMT).
To date, there is an abundance of evidence that has demonstrated improved clinical outcomes from GDMT in patients with HF with reduced ejection fraction (HFrEF) taking contemporary therapy. The 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HFSA) Guidelines for the Management of HF recommend initiating GDMT therapy consisting of four agents: (1) ACEI, ARB, or ARNI; (2) Beta-blocker (BB); (3) MRA; and (4) SGLT2i.[2] Titration of quadruple therapy to target dosing is associated with a significant reduction in morbidity and all-cause mortality among other GDMT therapy combinations, with an estimated cumulative effect of 73% relative reduction in all-cause mortality over two years.[3]
In 2018, the Change the Management of Patients with HF (CHAMP-HF) registry study found significant gaps in the utilization and titration of GDMT in HFrEF outpatients. Greene et al. noted that nearly half of their study population was receiving GDMT at recommended doses.[4] The common reasons for not receiving optimal GDMT were older age, female sex, African-American race, lack of provider awareness of GDMT guidelines, concerns about side effects, lack of access to care, and financial constraints.[4] To better identify gaps in care at University Health in San Antonio, TX, we aimed to evaluate outpatient prescribing patterns of GDMT and its clinical impact specifically in unfunded patients with HFrEF.
Objectives
The primary objective of this study was to evaluate the outpatient prescribing patterns of GDMT and its clinical impact in unfunded patients with HFrEF within a large county health system. The goal was to compare the prescribing rates within our institution to the national average and identify areas for improvement. The secondary objectives of this study were to assess rates of adherence to GDMT, hospitalization, overall mortality rates, the number of patients enrolled in medication assistance programs, and the number of follow-up patient care visits stratified by provider type and specialty.
MATERIALS AND METHODS
Setting
This was a single-center, retrospective, and observational study conducted at University Health, a tertiary care academic hospital with multiple outpatient clinics within San Antonio, TX. University Health is a non-profit hospital that is 340B eligible (a section of the Public Health Service Act that requires drug companies that participate in Medicaid to sell outpatient drugs at discounted rates to hospitals that serve low-income and indigent patients) and offers patients many cost savings programs to assist with clinical and medication costs.
Patient selection
A sample of unfunded patients who presented for outpatient follow-up between July 1, 2020, and March 31, 2022, were evaluated for study inclusion. Adult patients were eligible for inclusion if they had a chart diagnosis of chronic HF, a left ventricular ejection fraction (LVEF) of 40% or lower according to echocardiography performed within 12 months of enrollment, and were prescribed at least one oral medication for HF at the time of study enrollment {defined as either a: (1) loop diuretic; (2) ACEI, ARB, or ARNI; (3) BB; (4) MRA; or (5) SGLT2i}. Patients were unfunded if no insurance was documented within the electronic health record (EHR) or they were enrolled within University Health’s financial assistance program (a county program to cover costs for uninsured patients whose household income does not exceed 200% of the federal poverty level). Key exclusion criteria included patients <18 years of age, incarcerated, pregnant, currently receiving comfort care or enrolled in hospice, life expectancy <1 year, history of or plan for heart transplantation, left ventricular assist device, or dialysis.
Data collection
Data collected from an internal database report from the EHR included demographic and clinical data relevant to the study outcomes. Baseline demographic information for each subject included age, sex, race/ethnicity, and insurance status. Insurance status was defined as federal insurance (i.e., Medicaid and Medicare), commercial insurance, county financial assistance program, or uninsured. Clinical data for each subject included the first available body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), LVEF, B-type natriuretic peptide (BNP), serum creatinine (SCr), estimated glomerular filtration rate (eGFR), and potassium (K) during study duration. Prescription data for each subject included patients who ever had a prescription ordered for any GDMT agent, any GDMT agent at its target dose, or listed allergy to specific GDMT agents during the study duration. Medication adherence to GDMT was measured using the proportion of days covered (PDC) metric recommended by the National Quality Forum and Pharmacy Quality Alliance.[5] Adherence was defined as a PDC ≥80% for at least three GDMT agents. Clinical severity and engagement with the healthcare system were also recorded, including the total number of hospitalizations for any cause, 30-day HF readmissions, outpatient provider visits (for primary care or cardiology services), type of visit (telemedicine vs. in-person), and utilization of clinical pharmacy services.
Statistical analysis
The primary outcome was the utilization and titration to target dosing for triple GDMT therapy {consisting of: (1) ACEI, ARB, or ARNI; (2) BB; and (3) MRA}. Target dosing was defined per the 2022 AHA/ACC/HFSA HF guidelines. The secondary outcomes included factors associated with being prescribed triple GDMT.
Demographics, clinical characteristics, and outcomes of our cohort were summarized with descriptive statistics. Dichotomous variables were analyzed using the Chi-square or Fisher’s Exact test, as appropriate. All continuous variables were tested for normality using the D’Agostino-Pearson normality test. Normally distributed continuous variables were assessed using the t-test. Non-normally distributed continuous variables were assessed using the Mann-Whitney U test. A significant variable was defined as any variable that differed significantly between patients who were on triple GDMT therapy versus other combinations of GDMT (i.e., mono- or dual-GDMT therapy) with P < 0.05. JMP® software was utilized for data analysis.
RESULTS
Baseline cohort characteristics
Between July 1, 2020, and March 31, 2022, a total of 3219 patients presented to outpatient provider visits within the institution’s clinics. Of these, 232 patients met inclusion criteria (7.2%) [Figure 1]. The most common reason for exclusion was active insurance status (n = 2,655).
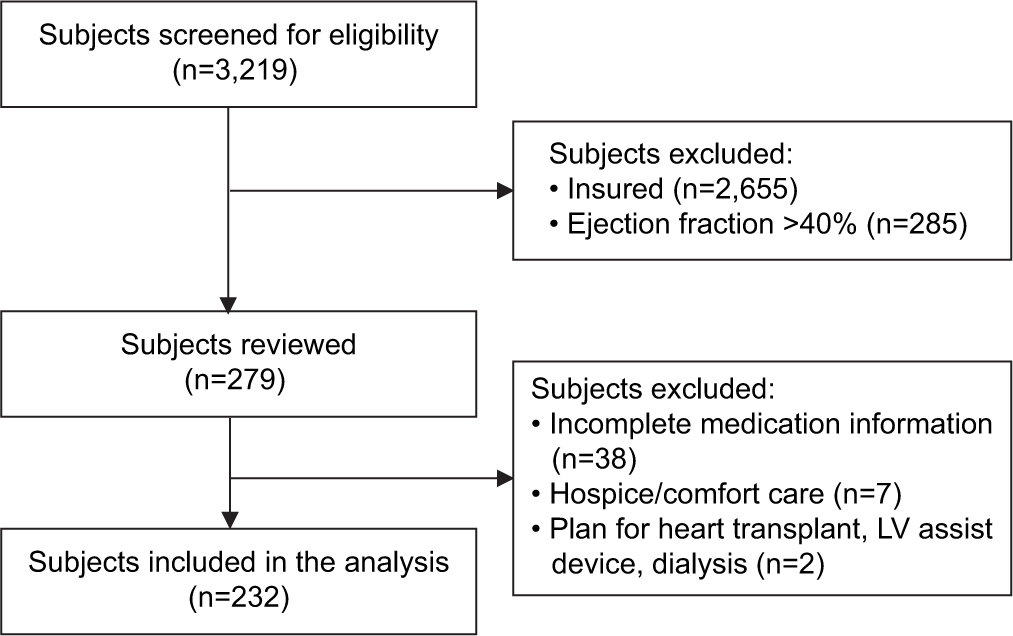
- Sample selection and attrition criteria.
Patients in the study were predominantly Hispanic/Latino (62.9%) middle-aged men (83%). Most patients were under the county’s financial assistance program (61.6%) and had a median age of 63 years (interquartile range [IQR] 58–69 years) [Table 1]. Patients had a median LVEF of 27% (standard deviation [SD] 8%), BNP of 401 pg/mL (IQR 117–808 pg/mL), SCr of 1.18 mg/dL (IQR 0.98–1.5 mg/dL), and a potassium level of 4.1 mmol/L (IQR 3.8–4.6 mmol/L). Vitals included a median SBP of 125 mmHg (110–139 mmHg), DBP of 74 mmHg (65–83 mmHg), HR of 80 beats/min (SD 14 beats/min), and BMI of 30.4 kg/m2 (IQR 25.2–36.4 kg/m2).
| Characteristic | All patients (n=232) | On triple GDMT (n=144) | Not on triple GDMT (n=88) | P-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 56 (49–61) | 56 (49–61) | 56 (49–60) | 0.630 |
| Male, n (%) | 191 (82) | 117 (81) | 74 (84) | 0.582 |
| Race, n (%) | ||||
| Caucasian | 150 (65) | 95 (66) | 55 (63) | 0.647 |
| African-American | 27 (12) | 14 (10) | 13 (15) | |
| Multiracial | 9 (4) | 5 (3) | 4 (5) | |
| Other | 46 (20) | 30 (21) | 16 (18) | |
| Ethnicity – Hispanic/Latino, n (%) | 146 (63) | 87 (60) | 59 (67) | 0.310 |
| Other HF medications, n (%) | ||||
| Loop diuretic | 191 (82) | 126 (88) | 65 (74) | 0.008 |
| Hydralazine/dinitrate | 10 (4) | 2 (1) | 8 (9) | 0.007 |
| Ivabradine | 4 (2) | 3 (2) | 1 (1) | 1.00 |
| Digoxin | 21 (9) | 15 (10) | 6 (7) | 0.354 |
| Vericiguat | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| SBP (mmHg), mean (SD) | 126 (21) | 125 (20) | 127 (22) | 0.374 |
| DBP (mmHg), mean (SD) | 75 (14) | 75 (12) | 75 (16) | 0.906 |
| HR (bpm), mean (SD) | 80 (14) | 81 (14) | 77 (12) | 0.131 |
| LVEF, mean (SD) | 30 (11) | 30 (11) | 31 (11) | 0.213 |
| BNP (pg/mL), median (IQR) | 401 (117–830) | 417 (124–881) | 253 (103–756) | 0.431 |
| SCr (mg/dL), median (IQR) | 1.18 (0.98–1.5) | 1.15 (0.97–1.36) | 1.31 (1–2.06) | 0.005 |
| eGFR (mL/min/1.73m2) | ||||
| Rate of >60 – n/total, n (%) | 100 (61) | 76/118 (64) | 24/47 (51) | 0.154 |
| Rate of 30–60 – n/total, n (%) | 55 (33) | 37/118 (31) | 18/47 (38) | |
| Rate <30 – n/total, n (%) | 10 (6) | 5/118 (4) | 5/47 (11) | |
| Potassium (mmol/L), median (IQR) | 4.1 (3.8–4.6) | 4.2 (3.9–4.5) | 4.1 (3.8–4.6) | 0.352 |
| BMI (kg/m2), median (IQR) | 30.4 (25.2–36.5) | 30.7 (26.9–37.1) | 29.8 (24.4–34.9) | 0.044 |
BMI (kg/m2), median (IQR) 30.4 (25.2–36.5) 30.7 (26.9–37.1) 29.8 (24.4–34.9) 0.044 BMI: Body mass index, SBP: Systolic blood pressure, DBP: Diastolic blood pressure, HR: Heart rate, LVEF: Left ventricular ejection fraction, BNP: B-type natriuretic peptide, SCr: Serum creatinine, eGFR: Estimated glomerular filtration rate, IQR: Interquartile range, GDMT: Guideline-directed medical therapy
By the end of the study period, triple GDMT was prescribed in 60.3% of patients, while 36.2% of patients were prescribed quadruple GDMT therapy. Among the study population, 48.3% were on an ACEI or ARB, 31% on an ARNI, 87.1% on a BB, 72.4% on an MRA, and 52.2% on a SGLT2i. Prescribing rates for target doses of ACEI/ARB, ARNI, BB, MRAs, and SGLT2i were 29.5%, 13.9%, 23.3%, 81.5%, and 90.1%, respectively [Table 2].
| GDMT agents | Rates of treatment | Rates of target dosing |
|---|---|---|
| ACEI/ARB/ARNI | ACEI/ARB: 48.3% ARNI: 31% |
ACEI/ARB: 29.5% ARNI: 13.9% |
| BB | 87.1% | 23.3% |
| MRA | 72.4% | 81.5% |
| SGLT2i | 52.2% | 90.1% |
| Patients on triple therapy (ACEI/ARB/ARNI+BB+MRA), no. (%) | 140 (60.3) | |
| Patients on quadruple therapy (triple+SGLT2i), no. (%) | 84 (36.2) | |
ACEI: Angiotensin-converting enzyme inhibitors, ARB: Angiotensin receptor blockers, ARNI: Angiotensin receptor/neprilysin inhibitor, BB: beta-blocker, MRA: Mineralocorticoid receptor antagonist, SGLT2i: Sodium-glucose cotransporter 2 inhibitor, GDMT: Guideline-directed medical therapy
Factors associated with GDMT utilization
Among patients prescribed triple GDMT therapy, 88% were hospitalized for any cause, 81% were hospitalized due to a cardiovascular (CV) cause (defined as myocardial infarction, stroke, or HFrEF exacerbation), and 14% of patients were readmitted within 30 days of hospital discharge. Conversely, 66% of patients not receiving triple GDMT therapy were hospitalized for any cause, 58% were hospitalized for a CV cause, and 12% of patients were readmitted within 30 days of hospital discharge. There was a significant difference between rates of hospitalization for any cause (P < 0.001) and CV causes (P < 0.001) between those on GDMT and those who were not. Furthermore, significantly higher rates of mortality were observed in patients not on GDMT compared to those that were (12% vs. 1% (P < 0.001)) [Table 3].
| Outcome | On triple GDMT (n=124) | Not on triple GDMT (n=59) | P-value |
|---|---|---|---|
| Medication adherence ≥80%, no. (%) | 56 (45) | 20 (34) | 0.174 |
| Hospitalization for any cause, no. (%) | 109 (88) | 39 (66) | 0.001 |
| Hospitalizations for any CV cause, no. (%) | 101 (81) | 34 (58) | 0.001 |
| 30-day HF rehospitalization, no. (%) | 17 (14) | 7 (12) | 0.730 |
| Death from any cause, no. (%) | 1 (1) | 7 (12) | 0.001 |
| Followed by transition of care pharmacist, no. (%) | 72 (58) | 22 (37) | 0.009 |
| Patient care visits | |||
| Visits to PCP, median (IQR) | 2 (1–5) | 3 (1–6) | 0.225 |
| Visits to cardiologist, median (IQR) | 3 (2–5) | 3 (1–4) | 0.125 |
| Any clinical pharmacy specialist visit, no. (%) | 24 (19) | 6 (10) | 0.117 |
| Any telemedicine visits, no. (%) | 63 (51) | 37 (63) | 0.131 |
| Enrolled in medication assistance program (MAP), no. (%) | 25 (20) | 6 (10) | 0.092 |
HF: Heart failure, PCP: Primary care providers, GDMT: Guideline-directed medical therapy, IQR: Interquartile range, CV: Cardiovascular
Of 144 patients receiving GDMT, only 56 patients (45.2%) had a PDC ≥ 80% for HF medications (P = 0.174). Over the 21-month follow-up period, patients were followed by many providers in the outpatient setting, including primary care providers (PCP), cardiologists, and clinical pharmacists. During the study duration, no differences were observed between the number of visits to either a PCP or a cardiologist (P = 0.225 and P = 0.125, respectively). In the GDMT group, there were more clinical pharmacist interactions compared to the non-GDMT group (58% vs. 37%, P = 0.0086).
DISCUSSION
While other studies have examined rates of GDMT prescribing in the outpatient setting, this study is one of the few to evaluate GDMT prescribing patterns solely in unfunded patients. Our results suggest that overall rates of GDMT prescribing and dose optimization in unfunded patients are low at our institution. These results are alarming given that our county’s financial assistance program offers medications at more affordable prices, which begs the question of why many patients still go without proper GDMT. The severity of suboptimal GDMT prescribing is highlighted in a study that evaluated the impact of GDMT in commercially insured people and found that inadequate GDMT confers a higher mortality rate.[6] These findings emphasize the need to identify other barriers to GDMT besides medication cost.
Many patient-specific factors can affect prescribing patterns, such as tolerability, lack of health literacy, and insurance status. Compared to our findings, among patients eligible for all classes of GDMT from the CHAMP-HF registry, 755 patients (22.1%) were simultaneously prescribed some dose of ACEI/ARB/ARNI, evidence-based BB, and MRA therapy, and 37 patients (1.1%) were simultaneously prescribed target doses of all three therapies. Our findings show that prescribing patterns and target dosing rates at our institution are much higher than the national average. These results were not something expected considering that most of our cohort fell in a lower healthy literacy category and all were uninsured. A population-based, retrospective study of newly diagnosed HFrEF patients in Olmsted County, Minnesota found that being seen in an HF clinic was independently associated with initiation of new GDMT across all classes.[7] This finding suggests that patients who seek care are more likely to be on GDMT than those who are resistant to seeking care. Moreover, this also may justify why our study found higher rates of hospitalization in patients on GDMT than those who were not.
Given the rising prevalence of HF and the fact that approximately 27 million Americans are uninsured, treatment of HF in unfunded patients needs further investigation.[8] Our study has several strengths that contribute to new findings in GDMT optimization literature. First, we had access to medication dispensing data from our institution’s system-based pharmacies. As a result, an accurate calculation of the PDC was used to assess medication adherence, similar to other HF GDMT utilization studies.[9] Although the study’s generalizability is limited to a low-income minority population, using patients from multiple clinics in San Antonio, TX increased the sample size and decreased the likelihood that provider- or clinic-specific practice biases could impact our findings.
The limitations of our study included a transition from one EHR system to another during the study period, which could have caused variability in data collection results and potentially underestimated the GDMT prescribing rates or achievement of target doses. In addition, the study period did not allow for a full assessment of GDMT quadruple therapy per the recent 2022 ACC/AHA/HFSA HF Guideline updates. During our study period, current guidelines (2017 ACC/AHA/FSA) only recommended triple therapy for GDMT. However, in the years between guideline updates, the use of quadruple therapy was increasingly becoming the standard of care due to the blooming evidence supporting the use of SGLT2i in HFrEF management. It is important to note that some patients in the study may have been receiving sacubitril/valsartan through a drug manufacturer’s patient assistance program (PAP) or may have filled GDMT prescriptions at an outside pharmacy. In either case, these patients’ medication adherence could not be accurately assessed due to PAP delivering medication directly from manufacturer to patient, or PDC was not available in our EMR. Moreover, 21.1% of patients initially identified for inclusion were lost to follow-up and were not included in the clinical outcomes analysis, which may result in an underestimation of our prescribing rates. Another limitation of this study is that dose titration information may not reflect changes made if patients were seen outside of our health system. While we are unable to quantify the number of patients affected by this, it is unlikely to be significant due to the discounted cost of medications offered to patients on the county’s financial assistance program.
The role of pharmacists in the management of HF is becoming increasingly important. As the population ages and the prevalence of HF increases, pharmacists will play a key role in helping patients achieve GDMT and improve clinical outcomes. Clinical pharmacists practicing under a collaborative practice agreement have the capability to titrate GDMT with closer follow-up than a PCP or cardiologist. According to Shah and colleagues, integrating clinical pharmacists into an HF clinic may be a workable approach at safety-net hospitals when a lack of medical staff and resources prevents prompt introduction and titration of GDMT.[10] For example, involving pharmacists within interdisciplinary teams increased GDMT use and optimization compared to teams without pharmacist involvement.[11] More so, Patil and colleagues found that pharmacist-led HF clinics significantly increased the proportion of patients who achieved triple and quadruple GDMT at 90 days, reduced hospitalization and ER visits related to HF, and improved quality of life.[12] Pharmacists can play a role in better understanding current prescribing patterns to help identify areas for improvement in HFrEF management. With this information, pharmacists can help improve patient outcomes, develop safer prescribing practices, and increase quality of care.
CONCLUSION
While our institution’s GDMT prescribing rates were higher than the national average, our findings illustrated significant gaps in proper GDMT utilization and dose titration for patients with HFrEF. Pharmacist involvement in GDMT dose titration within our institution was seldom, highlighting pharmacists’ potential to fill in these gaps of care for unfunded patients. Further research is warranted to explore the positive impact pharmacists can make on GDMT prescribing rates and achieving target dosing for quadruple therapy.
Ethical approval
The research/study was submitted to the Institutional Review Board at the University of Texas Health Science Center at San Antonio (HSC20220518EX), and it was determined to be exempt on 08/24/2022.
Declaration of patient consent
Patient’s consent was not required, as the study was retrospective.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: None.
References
- Heart disease and stroke statistics-2021 update: A report from the American Heart Association. Circulation. 2021;143:e254-e743.
- [CrossRef] [Google Scholar]
- 2022 AHA/ACC/HFSA guideline for the management of Heart Failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e263-e421.
- [CrossRef] [Google Scholar]
- Association of optimal implementation of sodium-glucose cotransporter 2 inhibitor therapy with outcome for patients with heart failure. JAMA Cardiol. 2020;5(8):948-951.
- [CrossRef] [PubMed] [Google Scholar]
- Medical therapy for heart failure with reduced ejection fraction: The CHAMP-HF registry. J Am Coll Cardiol. 2018;72(4):351-366.
- [CrossRef] [Google Scholar]
- PQA Adherence measures. Available from: https://www.pqaalliance.org/adherence-measures [Last accessed on 2023 Dec 21]
- [Google Scholar]
- Mortality and guideline-directed medical therapy in real-world heart failure patients with reduced ejection fraction. Clin Cardiol. 2021;44:1192-1198.
- [CrossRef] [PubMed] [Google Scholar]
- Guideline-directed medical therapy in newly diagnosed heart failure with reduced ejection fraction in the community. J Card Fail. 2022;28(10):1500-1508.
- [CrossRef] [PubMed] [Google Scholar]
- Health insurance coverage in the United States: 2019 Washington, DC: US Census Bureau; 2020. p. :5.
- [Google Scholar]
- Treatment initiation patterns, modifications, and medication adherence among newly diagnosed heart failure patients: A retrospective claims database analysis. J Manag Care Spec Pharm. 2016;22:561-571.
- [CrossRef] [PubMed] [Google Scholar]
- Integration of clinical pharmacists into a heart failure clinic within a safety-net hospital. J Am Pharm Assoc (2003). 2022;62:575-579.e2.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of pharmacist impact within an interdisciplinary inpatient heart failure consult service. Ann Pharmacother. 2019;53:905-915.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of pharmacist-led heart failure clinic on optimization of guideline-directed medical therapy (PHARM-HF) J Cardiovasc Transl Res. 2022;15:1424-1435.
- [CrossRef] [PubMed] [Google Scholar]







