Translate this page into:
Assessment of β-lactamase inhibition and antioxidant potential of ethanolic and aqueous leaf extracts of Irvingia gabonensis using GC-MS method
*Corresponding author: Caleb Joel Nwaogwugwu, PhD Department of Chemistry, Prairie View A and M University, Prairie View, Texas, United States. cjnwaogwugwu@pvamu.edu
-
Received: ,
Accepted: ,
How to cite this article: Okereke SC, Edom VC, Aaron CF. Assessment of β-lactamase inhibition and antioxidant potential of ethanolic and aqueous leaf extracts of Irvingia gabonensis using GC-MS method. Am J Pharmacother Pharm Sci 2024:14.
Abstract
Objectives:
This study aimed to investigate the antibiotic and antioxidant potential of aqueous and ethanol extracts from Irvingia gabonensis leaves, with a specific focus on their ability to inhibit β-lactamase enzyme activity. Both in vitro and in vivo approaches were employed to comprehensively assess the characteristics of the leaf extracts. Irvingia gabonensis, known for its medicinal properties, has been of interest due to its reported pharmacological activities. In the face of rising antimicrobial resistance, exploring natural sources for antimicrobial and antioxidant agents is crucial. This study builds upon existing knowledge by evaluating the phytochemical composition and potential therapeutic applications of Irvingia gabonensis leaf extracts.
Materials and Methods:
Gas chromatography-mass spectrometry (GC-MS) analysis was employed to identify the chemical components present in ethanolic leaf extracts. Antioxidant activities were assessed through various assays, including DPPH, FRAP, nitric oxide radical scavenging, and MDA scavenging assays. In vivo experiments involved the administration of different concentrations of leaf extracts to albino rats over a 28-day period. Blood samples collected were utilized for serum separation, enabling the analysis of antioxidant assays in vivo, including β-lactamase inhibition, lipid peroxidation (MDA), superoxide dismutase (SOD), and catalase (CAT) activity.
Results:
The ethanolic extracts exhibited a significant 65.96% inhibition rate against β-lactamase enzyme activity. Additionally, there was an increase in the concentrations of glutathione (GSH) and glutathione peroxidase (GPx), as well as heightened catalase (CAT) activity. Simultaneously, a decrease in malondialdehyde (MDA) concentration indicated the extracts’ potential to mitigate lipid peroxidation. These results highlight the robust antimicrobial and antioxidant properties of Irvingia gabonensis leaf extracts.
Conclusion:
Irvingia gabonensis leaf extracts demonstrate substantial potential as antimicrobial and antioxidant agents, emphasizing their role in combating antimicrobial resistance. The significant inhibition of β-lactamase enzyme activity and the modulation of key antioxidant markers suggest the extracts’ therapeutic relevance. This study contributes to the understanding of Irvingia gabonensis phytochemical composition and supports its potential application as an antioxidant supplement and β-lactamase inhibitor.
Keywords
Antioxidant
β-lactamase
Irvingia gabonensis
Phytochemical composition
Antimicrobial resistance
INTRODUCTION
Antimicrobials play a crucial role in combating harmful microorganisms by inhibiting or eliminating their growth. They are effective against a wide range of pathogens including Bacteria, Viruses, Fungi, and Protozoa. However, a growing concern is the emergence of resistance among pathogenic microorganisms toward commonly used antimicrobial agents. This poses a significant challenge in healthcare as it limits treatment options and increases the risk of infections becoming difficult to manage. Addressing antimicrobial resistance requires ongoing research and the development of alternative strategies to ensure effective control of infectious diseases and preserve the efficacy of antimicrobial treatments. The rapid emergence of resistance to recently introduced antimicrobial agents raises concerns that even new families of antimicrobial agents will have a limited shelf life. For these reasons, researchers are increasingly turning their attention to herbal remedies in their search for fresh ideas to create more effective medications for a variety of drug-resistant microbial strains.[1] To find new antimicrobial compounds, plant species must be screened for those that can produce new drugs.[2] Several bacteria and fungi have been shown to be inhibited by extracts of bush mango leaves and roots.[3,4]
A beta-lactam antibiotic is created by joining a thiazolidine ring to a beta-lactam ring and a side chain. Beta-lactam antibiotics are reportedly the most widely used antibiotics due to their high efficacy. However, recent reports have indicated that the most widespread infections are becoming more resistant.[1] One of the most clinically significant mechanisms of resistance for Gram-negative pathogenic bacteria is the production of beta-lactamase. Understanding the most prevalent beta-lactamases made by different pathogens can help with susceptibility analysis, treatment selection, and infection control procedures. Biologically, active compounds derived from medicinal plants have caught the attention of the scientific community due to the undesirable side effects associated with the indiscriminate use of synthetic antimicrobial medications.[5] Antioxidants present in plants have medicinal value, and interest in the exogenous plant antioxidants was first sparked by the discovery and subsequent isolation of ascorbic acid from plants.[6] Medicinal plants have antioxidant properties that may shield them from oxidative stress, which is a major contributing factor to the onset and progression of many fatal diseases, including neurodegenerative and cardiovascular disease.[7] Therefore, the present study was designed to investigate the antibiotic and antioxidant potentials of Irvingia gabonensis leaf extracts.
MATERIALS AND METHODS
Plant material
The leaf of I. gabonensis was obtained from Uturu, Abia State, Nigeria. The plant was identified and authenticated by a taxonomist and deposited at the institution Herbarium at the Department of Plant Science and Biotechnology at Abia State University in Uturu. Voucher specimens were numbered ABSU/PSB/234.
Sample preparation
Extraction of I. gabonensis leaf extracts using water and ethanol solvents
Water solvent extraction
Fresh I. gabonensis leaves were cleaned and separated to ensure purity. The leaves were dried in a shaded oven to retain active compounds. Once dried, the leaves were finely ground for increased surface area. For extraction, 200 g of the ground leaves were mixed with 1 L of distilled water in a container. The mixture was shaken and allowed 24 h to facilitate compound extraction. After shaking, the mixture was filtered, and the filtrate was concentrated through evaporation on a water bath at 40°C. The concentrated solution’s weight was recorded as the aqueous extract of I. gabonensis leaves, enriched with water-soluble compounds.
Ethanol solvent extraction
Two hundred grams of ground leaves were placed in a container, and 95% ethanol solvent was added to cover the plant material. The container was sealed and left to soak for a designated period. The mixture was then filtered to obtain an extract solution containing ethanol-extracted compounds. This solution underwent evaporation on a controlled water bath at 40°C to remove ethanol, yielding a concentrated extract. The weight of the concentrated extract was recorded, representing the compounds extracted from the leaves using ethanol as the solvent.
Both extraction methods capture distinct compounds, contributing to the potential antimicrobial and antioxidant properties of the respective extracts
Preparation of plant extract for gas chromatography-mass spectrometry (GC-MS) analysis
The dried extract of I. gabonensis leaves was dissolved in absolute ethanol at a concentration of 1 mg/mL. A volume of 10 mL of the filtered ethanolic extract sample was then prepared for GC-MS analysis.
GC-MS analysis
GC-MS analysis was conducted utilizing the GC-MS QP2010 ultra instrument. The analytical system was outfitted with an InertCap 5 MS/NP column sourced from GL Sciences. Before analysis, the mass spectrometer underwent calibration and tuning in accordance with the manufacturer’s specifications.
The mass spectrometer operated in electron impact mode at 70 eV. A filtered sample volume of 1 μL was injected into the GC injection port in split mode (25:1, v/v) at a temperature of 230°C. The mobile phase, consisting of an inert carrier gas (helium, He), exhibited a linear velocity of 39 cm/s and a flow rate of 1.12 mL/min. The column temperature was initially set at 60°C for 1 min and then ramped up at a rate of 15°C/min until reaching a maximum temperature of 300°C, with a 3-min hold time at each increment. The transfer line and ion source temperatures were maintained at 250°C and 200°C, respectively. As the eluate passed through the GC component, electron ionization at 0.94 kV generated ions. The recorded spectra were acquired at a scan rate of 10,000 u/s within the mass (m/z) range of 85–500. The data were captured and displayed on a visual display device. To identify the various chemical components, an online database or library search was conducted, involving a direct comparison of retention time, mass spectral information, and fragmentation patterns with standardized compounds in the national institute of standards and technology library.
Experimental animal
For the study, 50 albino rats (25 males and 25 females), weighing between 100 and 150 g each were purchased from the Animal house of the Biochemistry Department at Abia State University in Uturu, Nigeria. Before the experiment started, the rats were given 14 days to acclimatize. The rats were housed in typical environmental settings with 1 h/12 h light/dark cycles, humidity between 35% and 60%, and temperatures between 25°C and 28°C. In addition, they were given unlimited access to water and were fed regularly with standard rat food. The study strictly complied with the ethical principles of the World Health Organization’s good laboratory practice regulations from 1998 and the United States’ guidelines for using experimental animals.[8]
Experimental design
Twenty-five albino rats of the same stock assumed healthy, both male and female, were divided into five groups of five at random. Group 1 served as the control and was allowed access to both water and food ad libitum. Group 2 received 400 mg/kg of the aqueous leaf extract. Group 3 received 800 m/kg of the aqueous leaf extract, Group 4 received 400 mg/kg of the ethanol leaf extract, and Group 5 received 800 mg/kg of the ethanol leaf extract of I. gabonensis. Throughout the course of the study, the animals received daily doses for 28 days.
Blood collection
After 28 days of treatment with the aqueous and ethanolic I. gabonensis extract, the animals were fasted overnight, anesthetized with chloroform, and sacrificed. Blood from each animal was collected by cardiac puncture into dry test tubes. The blood sample was divided into two groups and was centrifuge at 3000 rpm for 20 min. Serum was separated from the clot with a Pasteur pipette into sterile sample test tubes for in vivo antioxidants assay.[9]
Beta-lactamase inhibition test
The objective of the beta-lactamase inhibition test was to evaluate the potential inhibitory effects of crude plant extracts on the hydrolysis of nitrocefin by β-lactamase. The method involved the preparation of plant extract solutions, incubation with β-lactamase, and subsequent measurement of enzyme activity using a plate reading spectrophotometer.
Preparation of crude plant extract solutions
Crude plant extracts were prepared at a concentration of 6 mg/mL. Each extract was dissolved in 50 mM sodium phosphate buffer with a pH of 7.0. This buffered solution facilitated the stability of the extracts and maintained a consistent pH for the assay.
Setup of microdilution plates
Microdilution plates were used to conduct the assay. Each well of the plate received 80 μL of the respective plant extract solution. Control wells contained only the phosphate buffer without any plant extract. In addition, 10 μL of diluted β-lactamase was added to each well.
Pre-incubation
The microdilution plates were incubated for 30 min at room temperature. This pre-incubation step allowed for any potential interactions between the plant extracts and β-lactamase.
Start of assay
To initiate the assay, 10 μL of nitrocefin (at a concentration of 500 μM) was manually added to each well. This chromogenic substrate is hydrolyzed by β-lactamase, resulting in a color change that can be measured spectrophotometrically.
Measurement of enzyme activity
The activity of β-lactamase was monitored using a plate reading spectrophotometer at a wavelength of 482 nm. The measurements were taken continuously for a minimum of 30 min to capture the change in absorbance over time.
Calculation of residual activity
The initial velocity of β-lactamase catalyzed hydrolysis was calculated for each well as absorbance units per minute (AU/min). The residual activity of the enzyme was determined using the following formula:
Residual Activity (%) = (Initial Velocity/Initial Velocity Blank) × 100
Here, “Initial Velocity Blank” refers to the initial velocity of β-lactamase hydrolysis in the absence of the plant extract. This served as the baseline control.
Calculation of inhibition
The percentage of inhibition exhibited by the plant extract was calculated using the formula:
Inhibition (%) = 100–Residual activity
This provided a measure of how effectively the plant extract inhibited the hydrolysis of nitrocefin by β-lactamase.
Determination of the in vitro antioxidant activities of I. gabonensis leaf extract using following
2, 2-diphenyl-1-picrylhydrazyl (DPPH) photometric assay
The free radical scavenging activity of both aqueous and ethanolic extract was analyzed by DPPH assay using spectrophotometer.[10] Each of the test aqueous and ethanolic extracts (2 mL) at different concentrations (50 g/mL, 100 g/mL, 200 g/mL, 400 g/mL, and 800 g/mL) was mixed with 0.5 mM DPPH (in 1 mL of ethanol) in a cuvette. One milliliter of ethanol plus 2.0 mL of the extract was used as the blank, while 1.0 mL of the 0.5 mM DPPH solution plus 2.0 mL of ethanol was used as the negative control. Ascorbic acid (vitamin C) was used as reference standard.[11]
The absorbance at 517 nm was taken after 30 min of incubation in the dark at room temperature. The concentrations were prepared in triplicates and the percentage antioxidant activity calculated as follows:
Ferric reducing antioxidant power (FRAP) activity assay
The Benzie and Strain method[12] was used to calculate the extracts’ FRAP. In a nutshell, 3.1 g of sodium acetate trihydrate were dissolved in 16 mL of glacial acetic acid, pH 3.6, to create the acetate buffer (Reagent A). Reagent B was prepared by dissolving 10 mM of 2, 4, 6-tripyridyl-s- triazine (TPTZ) in a 40 mM HCl solution. A 20 mM solution of FeCl3•6H2O served as reagent C. Reagent A, Reagent B, and Reagent C were mixed in a ratio of 10:1:1 to form the FRAP reagent. To conduct the experiment, 80 mL of plant extract was combined with 3.6 mL of the FRAP reagent, 0.4 mL of distilled water, and another 3.6 mL of the FRAP reagent. The reaction mixture was then incubated at 37°C for 10 min. The FRAP value of the extracts was determined by extrapolating from a standard FeSO4•7H2O and expressed as mM Fe++ equivalents, following the methodology described by Noreen et al.[12]
Nitric oxide radical scavenging activity
Nitric oxide radical scavenging activity was assessed following the methodology outlined by Farhan et al.[13] The reaction mixture consisted of a solution of sodium nitroprusside with a concentration of 5 mmol/L in phosphate-buffered saline at pH 7.4, along with various concentrations of the ethanolic extract (ranging from 250 mg/mL to 2500 mg/mL) prepared in methanol. The mixture was incubated for 30 min at a temperature of 25°C. After the incubation period, a portion of the incubated solution (1.5 mL) was mixed with 1.5 mL of Griess reagent containing 0.1% N-1-naphthyl ethylene diamine dihydrochloride (NED), 1% sulfanilamide, and 2% phosphoric acid. Quercetin was utilized as the standard drug. The presence of nitrite ions was diazotized with sulfanilamide and coupled with NED, resulting in the formation of a pink chromophore. The absorbance of the chromophore was measured spectrophotometrically at 546 nm against a blank. The blank comprised all the reactants except for the extract.
All tests were conducted in triplicate to ensure accuracy and reliability of the results.
Where A0= control reaction absorbance (blank) and A1= extract or quercetin absorbance.
Malondialdehyde (MDA) scavenging activity
The method for measuring MDA scavenging activity was adapted from the protocol described by M. Senthilkumar, N. Amaresan, A. Sankaranarayanan.[14] In this study, a reaction mixture with a final volume of 1.0 mL was prepared, consisting of 2.0 mL of the trichloroacetic acid (TCA)-thiobarbituric acid (TBA)-HCl reagent (15% [w/v] TCA, 0.375% [w/v] TBA, and 0.25 N HCl) and the plant extract (at concentrations ranging from 50 mg/mL to 250 mg/mL). The reaction mixture was then heated on a water bath at 90°C for 10 min, cooled, and centrifuged at 10,000 rpm for 10 min to remove the TCA precipitate, resulting in a light pink-colored supernatant containing MDA. Ascorbic acid was used as a reference drug in the experiment. The MDA content in each sample was determined by measuring the absorbance of the clear supernatant at 532 nm against a reference blank. The experiment was repeated 3 times, and the percentage of lipid peroxidation (LPO) inhibition was calculated using the formula:
Where A0= control reaction absorbance (blank) and A1 = extract or ascorbic acid absorbance. MDA: Malonaldehyde
LPO in serum
Three test tubes with the labels “test,” “serum,” and “blank” were filled with 10 mL of serum and 10 mL of distilled water. Then, 0.5% NaOH solution was added to 0.5 mL of 1% TBA and 0.25 mL of 25% TCA. The resulting mixture was cooked for 40 min in a water bath before being cooled in cold water. After the solution had cooled, 0.1 mL of 20% sodium dodecyl sulfate was added and carefully mixed. With reference to a blank, the solution’s absorbance was measured using a spectrophotometer at 532 nm and 600 nm.[15]
MDA: Malonaldehyde, abs: Absorbance.
Determination of superoxide dismutase (SOD) activity
The reaction mixture consisted of 3 mL of serum, 1.9 mL of phosphate buffer (pH 7.8), 1 × 10–2 M methionine, 16.8 × 10–5 M NBT, and 1.17 × 106 M riboflavin. The solution was placed in a 10 mL beaker and exposed to illumination from a 15 W fluorescent lamp for 10 min inside an aluminum foil-lined box. A control sample without the enzyme source was also included. The absorbance of the solution was measured at 560 nm, and the obtained value was expressed in units per gram of hemoglobin (units/GmHb).[16]
Estimation of catalase (CAT) activity
In a test tube, 0.9 mL of distilled water and 0.1 mL of plasma were combined. To this mixture, 2 mL of H2O2 and 2 mL of phosphate buffer were added. In addition, 2 mL of dichromate acetic acid reagent was added to a separate 1 mL portion of the mixture to initiate the reaction. The absorbance of the reaction was measured every 30 s for a duration of 2 min. The unit of measurement for CAT activity is U/mL of plasma, which represents the micromoles of H2O2 used per second.[17]
The calculation for CAT activity (U/mL) is as follows:
Catalase activity (U/mL) = 0.23 × log (Abs 1/Abs 2)/0.00693
Determination of the glutathione (GSH) concentration
0.1 mL of blood, 0.9 mL of purified water, and 0.02 mL of sodium sulfate were combined in a beaker. After giving the combination a good shake, the mixture was left to stand at room temperature for 2 min. The beaker was then filled with 0.02 mL of 20% lithium sulfate, 0.22 mL of 20% sodium carbonate, and 0.22 mL of phosphor-18-tungstic acid. After shaking the mixture, 4 min were spent watching the color developed. After 10 min, the solution’s absorbance at 680 nm was measured after the addition of 2.5 mL of 20% sodium sulfate. 0.1 mL of water was used in place of blood to create an experiment that was completely blank. By creating a standard cysteine, the GSH concentration was calculated.[18]
GSH: Glutathione, abs: Absorbance. blk: Blank
Statistical analysis
The results in vivo and in vitro were expressed as mean ± Standard deviation and subjected to a paired samples t-test and statistical significance obtained at P < 0.05. The data generated from the animal study were analyzed with a oneway analysis of variance and the means compared with a Duncan multiple range comparison test using a statistical products and service solutions version 22 and statistical significance established 95% confidence level (P < 0.05).
RESULTS
Result of GC-MS analysis
Irvingia gabonensis ethanol extract’s GC-MS spectrum analysis identified a wide range of chemical components, most of which were fatty acids. Oleic acid (12.97%), linoelaidic acid (24.92%), and 6-octadecenoic acid (10.94%) are the main constituents. In addition, different amounts of other fatty acids including octadecanoic acid, stearic acid, and linoleic acid were found. Additionally, trichloroacetic acid, pyrimidine-4,6 (3H,5H)-dione, and 5-octadecene were found to be minor compounds. These results imply that the rich chemical profile of Irvingia gabonensis ethanol extract may be responsible for some of its biological and pharmacological characteristics.
Results of in vitro assessment of β-lactamase inhibition
The β-lactamase inhibition assay is a type of biochemical assay that is used to determine the ability of a substance to inhibit the activity of β-lactamase enzymes. β-lactamases are enzymes produced by some bacteria that can break down and inactivate β-lactam antibiotics, such as penicillin and cephalosporins. In the case of the aqueous leaf extract of
I. gabonensis, the β-lactamase inhibition assay showed that the extract had a % inhibition of 80.96%. This means that the extract was able to inhibit the activity of β-lactamase enzymes by 80.96%.
Results of in vitro antioxidant potential of aqueous and ethanol extracts of I. gabonensis leaves
The study used three assays—Nitric Oxide Scavenging Activity, DPPH Scavenging Activity, and MDA Scavenging Activity—to assess the antioxidant potential of Irvingia gabonensis leaf extracts. The findings were displayed as the concentration of scavenged compounds per milliliter of extract or as a percentage of inhibition, with error bars denoting standard deviation. At p < 0.05, significant differences were indicated between the ethanol and aqueous extracts. Overall, the results point to Irvingia gabonensis’ possible antioxidant qualities; variations are related to the phytochemical composition and extraction techniques.
Results of in vivo antioxidant potential of aqueous and ethanol extracts of I. gabonensis leaves
The effect of Irvingia gabonensis leaf aqueous and ethanol extracts on antioxidant parameters in albino rats, both male and female, was examined in this study. The following parameters were measured: malonaldehyde (MDA), superoxidase dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GPx). The administration of extract raised GSH and GPx levels, especially at higher doses, according to the results. SOD levels responded differently to different doses; in particular, in females, they increased at higher doses while decreasing at lower doses. Generally speaking, CAT levels increased with extract consumption, especially at higher dosages; the ethanol extract showed the highest levels at 800 mg/kg. MDA levels dropped, especially at higher doses and in both genders, suggesting decreased oxidative stress and lipid peroxidation. Both extracts demonstrated antioxidant qualities overall, with the ethanol extract having somewhat greater effects.
DISCUSSION
The molecular formula, weight, and peak area of each of the bioactive compounds found in I. gabonensis’s GCMS analysis are listed in Table 1. The analysis revealed the presence of various bioactive compounds in I. gabonensis leaf extract, such as 9, 12-octadecadienoic acid, n-hexadecanoic acid, 9,12-Octadecadienoic acid, methyl ester, oleic acid, linolelaidic acid, dodecanoic, myristic acid, and other significant volatile compounds with antiulcer, anti-inflammatory, anti-arthritic, antidiabetic, hypolipidemic, and cytotoxic properties [Figure 1]. These compounds justify the use of the plant leaf for various treatments by regional traditional healers. Organic component 9, 12-octadecadienoic acid, which is present in some tropical vegetable oils, aids in the treatment of neurodegenerative diseases.[19,20] n-Hexadecanoic acid has antioxidant, 5-alpha-reductase inhibitor, anti-fibrinolytic, hemolytic, antimicrobial activity, hypocholesterolemic nematicide, pesticide, antiandrogenic flavor, and hemolytic properties.[21] 9,12-Octadecadienoic acid, methyl ester has anti-inflammatory, anti-arthritic, hepatoprotective, antiandrogenic, hypocholesterolemic, nematicide, 5-alpha-reductase inhibitor, antihistaminic, anti-coronary, insectifuge, antieczemic, and anti-acne properties.[20,22] Oleic acid is an omega-9 fatty acid and is most commonly used for preventing heart disease and reducing cholesterol. Linolelaidic acid is an omega-6 trans-fatty acid and is a cis–trans isomer of linoleic acid. The omega-6 fatty acids have two main roles in the body: (1) they act as structural components of membranes influencing membrane function, and (2) they act as precursors of eicosanoids, which modulate renal and pulmonary function, vascular tone, and inflammatory responses.[22] Dodecanoic also known as lauric acid may be helpful to prevent microbial infection and manage the balance and distribution of bacteria in the human gut microbiota due to its wide range of antimicrobiological activities against enveloped viruses and different bacteria.[23] Myristic acid, also known as tetradecanoic acid, is an essential oil that can be used as a natural preservative to safeguard the nutritional value of food products from the damaging effects of free radicals. It also has strong antioxidant and radical-scavenging potential.[24] Other significant volatile compounds with antiulcer, anti-inflammatory, anti-arthritic, antidiabetic, hypolipidemic, and cytotoxic properties include esters, alkanes, and phenolic compounds.[25]
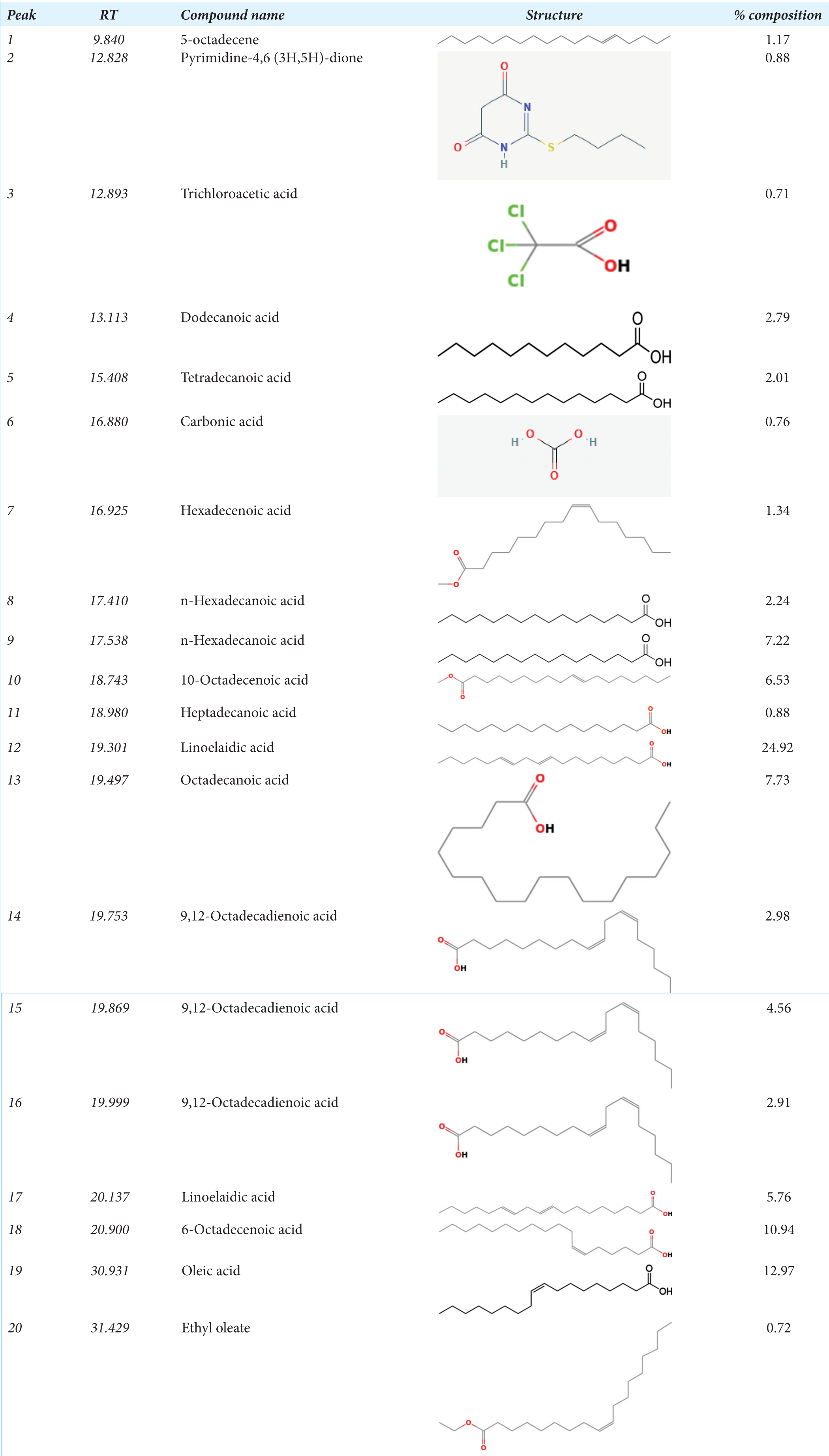 |
GC-MS: Gas chromatography-mass spectrometry, RT: Retention time
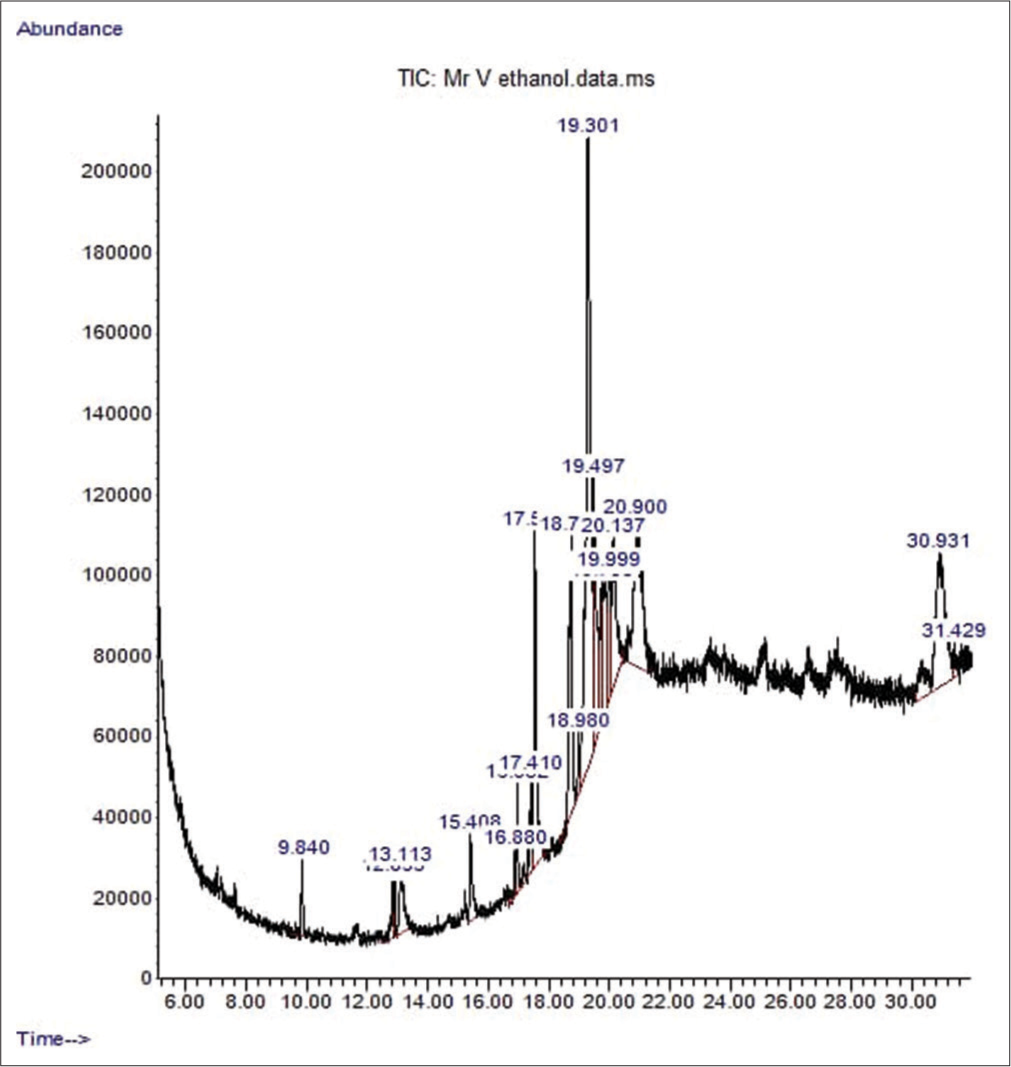
- The chromatogram showing the peak of the various chemical constituents of Irvingia gabonensis leaf extract. TIC: Total ion current.
The study investigated beta-lactamase inhibitors from the aqueous extracts of I. gabonensis leaves. The results showed that the leaves of I. gabonensis contain natural beta-lactamase enzyme inhibitors that could be used to prevent bacterial beta-lactamases from inactivating beta-lactam antibiotics [Figure 2]. Beta-lactamase inhibitors are an important class of compounds that are used to prevent bacterial beta-lactamases from inactivating beta-lactam antibiotics. In this study, we aimed to investigate the potential beta-lactamase inhibitors present in aqueous extracts of I. gabonensis leaves. Our simple blueprint involved using an aqueous extract of these leaves to screen for naturally occurring beta-lactamase enzyme inhibitors. The results showed that the leaves of I. gabonensis exhibited a high percentage inhibition of 80.96% and residual activity of 19.04%. The findings indicated that the leaves of I. gabonensis displayed a significant inhibitory effect, with a substantial inhibition percentage of 80.96% and residual activity of 19.04%. This suggests the presence of compounds capable of impeding the growth of microorganisms, specifically bacteria. These results align with prior studies documented in the literature, such as the research conducted by Ajaiyeoba[26], which investigated the phytochemical composition and antimicrobial properties of crude methanol extracts from wild mango leaves and roots.
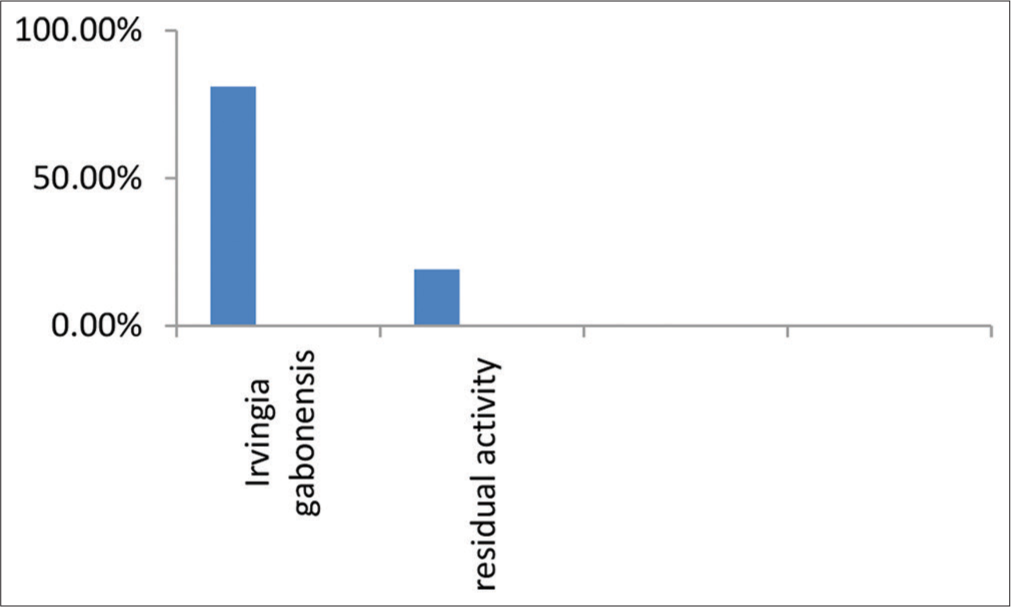
- β-lactamase inhibition assay by aqueous extract of Irvingia gabonensis leaves.
Furthermore, the study evaluated the impact of different doses of I. gabonensis leaf extracts on five antioxidant parameters, namely, GSH, glutathione peroxidase (GPx), SOD, CAT, and MDA. The ability of the extracts to alleviate oxidative stress in rats was determined in this study by measuring MDA, GSH concentration, and GPx and CAT activity in the liver. GSH is a non-enzymatic tripeptide biological antioxidant found in the liver that protects cellular proteins from reactive oxygen species (ROS) produced by a variety of processes. GSH deficiency is linked to increased LPO. GPx is also involved in the detoxification of hydroperoxides. It catalyzes the reaction of hydroperoxides with reduced GSH to produce GSH disulfide (GSSG) and the hydroperoxide reduction product.[27] CAT is an antioxidant enzyme that acts as a catalyst for the conversion of hydrogen peroxide to oxygen and water. It nullifies the effect of hydrogen peroxide that is present intracellularly. MDA is a by-product of LPO. Increased levels of LPO as measured by MDA have been linked to a variety of disease conditions and pathological states. In this study, the GSH and GPx concentrations, as well as CAT activities, increased significantly while the MDA concentration decreased significantly in all male and female rats treated with aqueous and ethanol extracts at 400 and 800 mg/kg body weight, respectively. The effect of aqueous and ethanol leaf extracts of I. gabonensis on the antioxidant parameters of male and female albino rats is presented in Table 2. The results presented in Table 3 showed that both the aqueous and ethanol leaf extracts of I. gabonensis significantly increased all the antioxidant parameters measured at all doses, except for MDA levels, which were significantly decreased at all doses. Moreover, Table 2 presented the effect of aqueous and ethanol leaf extracts of I. gabonensis on the antioxidant parameters of male and female albino rats.
| Treatment groups | Control | Aqueous extract (400 mg/kg) | Aqueous extract (800 mg/kg) | Ethanol extract (400 mg/kg) | Ethanol extract (800 mg/kg) |
|---|---|---|---|---|---|
| GSH (u/L) | 40.53±0.87a | 43.70±0.46b | 43.20±1.18b | 45.73±0.71c | 46.93±0.51c |
| GPx (u/L) | 46.37±0.42a | 48.23±0.81b | 50.50±0.36c | 45.93±0.61a | 52.43±1.15d |
| SOD (u/L) | 37.57±0.06a,b | 35.63±0.80a | 35.43±0.90a | 36.53±1.91a,b | 38.70±1.47b |
| CAT (u/L) | 21.30±0.95a | 23.50±1.65a,b | 24.87±1.65b,c | 26.43±1.12c,d | 27.47±0.87d |
| MDA (mmol/L) | 0.45±0.01c | 0.40±0.01c | 0.32±0.01a | 0.31±0.02a | 0.29±0.01a |
Values are presented as mean±standard deviation (n=3); and values with different superscripts are significantly (P<0.05) different from any paired mean across the row. GSH: Glutathione, GPx: Glutathione peroxidase, SOD: Superoxide dismutase, CAT: Catalase, MDA: Malondialdehyde
| Treatment groups | GSH (u/L) | GPx (u/L) | SOD (u/L) | CAT (u/L) | MDA (mmol/L) |
|---|---|---|---|---|---|
| Control | 43.73±1.11a | 48.10±1.54a | 36.67±1.78a | 20.17±0.87a | 0.42±0.03c |
| Aqueous extract (400 mg/kg) | 45.43±0.55a,b | 49.57±1.10a | 36.57±0.70a | 23.97±0.47b | 0.38±0.02b |
| Aqueous extract (800 mg/kg) | 46.13±1.02b,c | 52.93±1.40b | 38.23±0.61a | 23.97±1.39b | 0.33±0.02a |
| Ethanol extract (400 mg/kg) | 47.73±1.29c,d | 48.33±1.21a | 36.67±1.08a | 25.20±0.26b | 0.36±0.02a,b |
| Ethanol extract (800 mg/kg) | 49.00±0.66d | 54.93±1.21b | 38.57±1.97a | 25.43±1.48b | 0.33±0.03a |
Values are presented as mean±standard deviation (n=3); and values with different superscripts are significantly (P<0.05) different from any paired mean in each column. GSH: Glutathione, GPx: Glutathione peroxidase, SOD: Superoxide dismutase, CAT: Catalase, MDA: Malondialdehyde
The results demonstrated that the aqueous leaf extract significantly increased GSH and CAT activity at both doses compared to the control group. In contrast, the ethanol leaf extract significantly increased all antioxidant parameters measured at both doses compared to the control group. The study concluded that the I. gabonensis extracts have potential antioxidant effects in both male and female albino rats, with the ethanol extract showing a more significant increase in all the antioxidant parameters measured. Further studies are needed to determine the safety and efficacy of I. gabonensis extracts in humans.
Oxidative stress is a result of an imbalance favoring prooxidants, which leads to overproduction of ROS, the main catalyst for bimolecular oxidation. Antioxidants play a crucial role in mitigating the effects of free radicals by reacting directly with, disguising from, or competing with substrates whose terminal electron acceptor is molecular oxygen.[28,29] Natural antioxidants from medicinal plants are preferred alternatives to many commercially available synthetic antioxidants, which have been shown to be toxic and carcinogenic.[30] Herbal remedies are effective therapeutic tools for treating and preventing degenerative diseases caused by oxidative stress.
In this study, we investigated the in vitro antioxidant and free radical scavenging activities of the ethanol leaf extract of I. gabonensis [Figure 3]. The results demonstrated notable DPPH radical scavenging activity, ferric reducing capacity, nitric oxide scavenging activity, and LPO (MDA) scavenging activity at various concentrations (50, 100, 200, 400, and 800 μg/mL). The reducing abilities of the I. gabonensis leaf extract were dose-dependent and comparable to the reference compound, ascorbic acid (Vitamin C). These findings suggest that I. gabonensis may be a potential source of natural antioxidants for use in the prevention and treatment of oxidative stress-related degenerative diseases [Figures 4-6].[31,32]
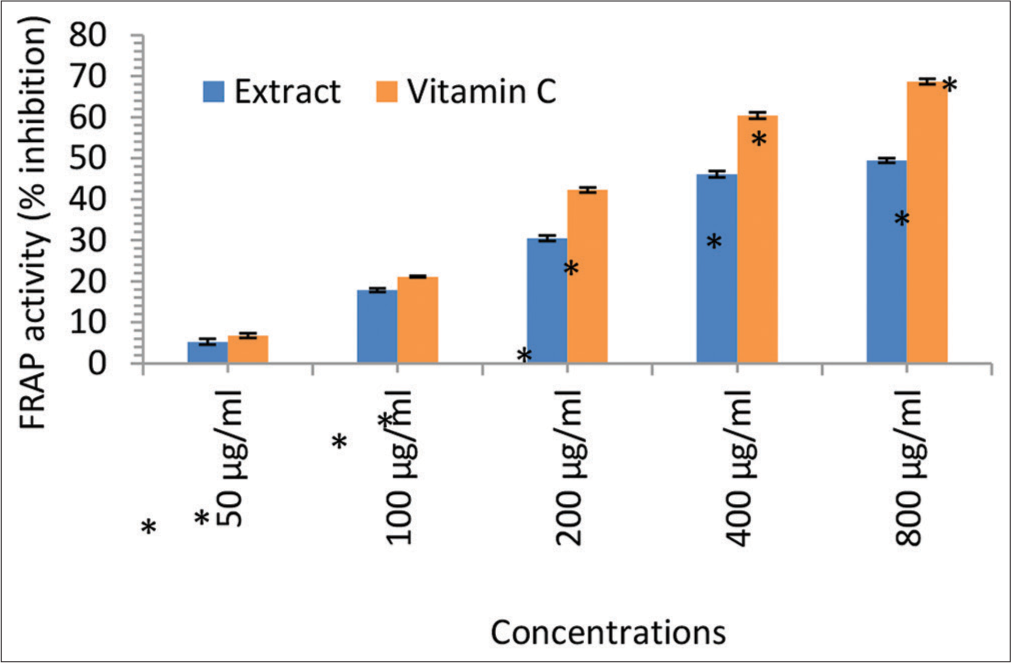
- Ferric reducing antioxidant power (FRAP) activity (% inhibition) of the Irvingia gabonensis leaf extract. Bars are presented as mean ± standard deviation (n = 3); and paired bars with different asterisks (* and **) are significantly different at P < 0.05.
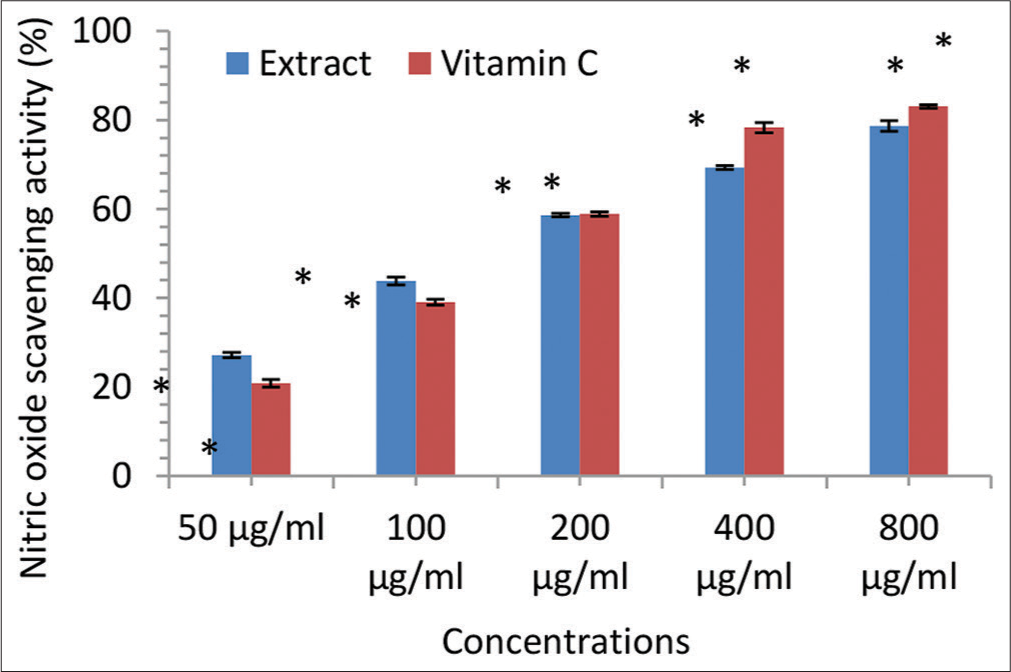
- Nitric oxide scavenging activity (% inhibition) of the Irvingia gabonensis leaf extract. Bars are presented as mean ± standard deviation (n = 3); and paired bars with different asterisks (* and **) are significantly different at P < 0.05.
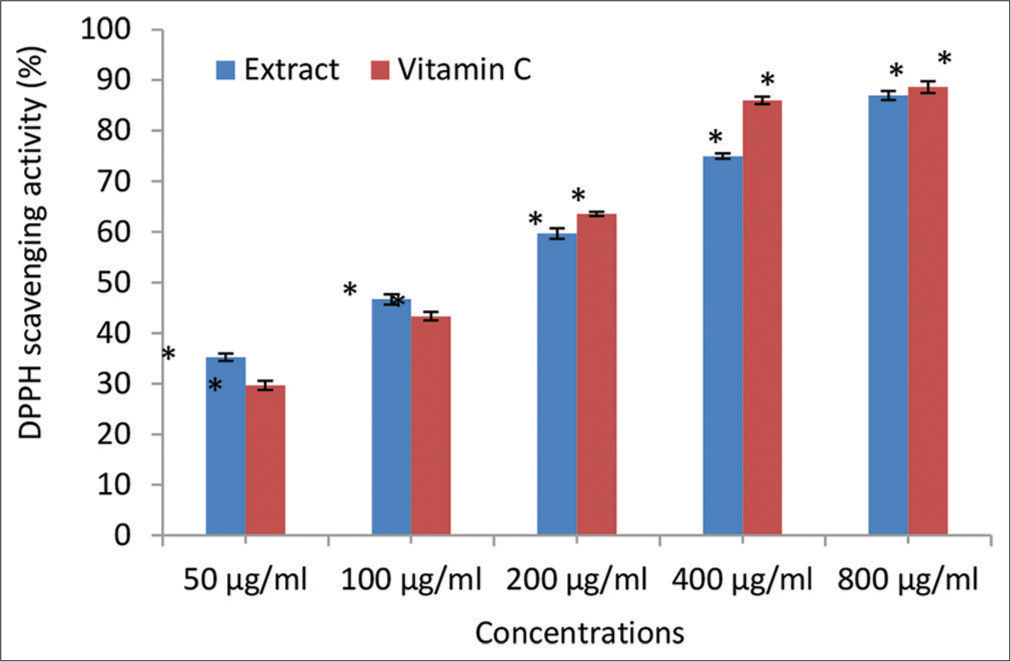
- 2, 2-diphenyl-1-picrylhydrazyl (DPPH) scavenging activity (% inhibition) of the Irvingia gabonensis Leaf Extract. Bars are presented as mean ± standard deviation (n = 3); and paired bars with different asterisks (* and **) are significantly different at P < 0.05.
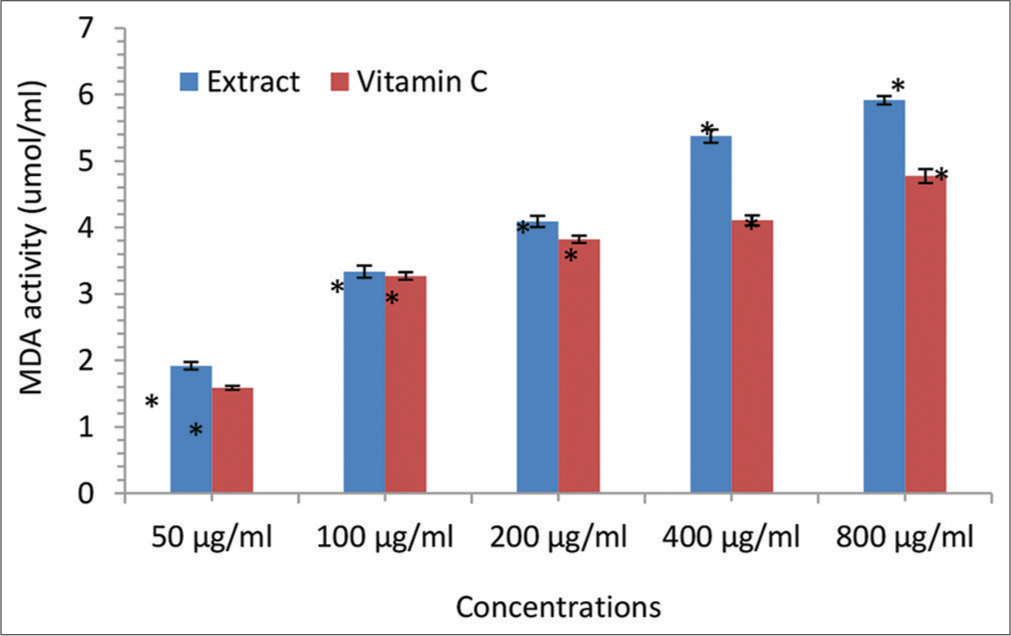
- Malondialdehyde (MDA) scavenging activity (μmol/mL) of the Irvingia gabonensis leaf extract. Bars are presented as mean ± standard deviation (n = 3); and paired bars with different asterisks (* and **) are significantly different at P < 0.05.
CONCLUSION
The in vivo antioxidant activity outcomes demonstrated that oral administration of extracts increases plasma antioxidant capacity against the DPPH radical and improves the antioxidant status of the liver and kidney by lowering the concentration of MDA and raising the rate of reduced GSH and CAT activity. The plant leaf extract’s antioxidant (in vitro) and beta-lactamase inhibitory potentials, along with its pharmacologically bioactive constituents, support its potential use as an antioxidant supplements and beta-lactamase inhibitor.
Ethical approval
The study strictly complied with the ethical principles of the World Health Organization’s good laboratory practice regulations from 1998 and the United States’ guidelines for using experimental animals.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
None.
References
- Antimicrobial activities of Irvingia gabonensis leaf against diarrhoea causing agents. Int J Adv Eng Res Sci. 2019;6:237-43. doi:10.22161/ijaers.6.3.31
- [CrossRef] [Google Scholar]
- Antibacterial activity of the leaf and stem bark of Irvingia gabonensis (Bush mango) against Escherichia coli and Staphylococcus aureus. Glob J Pharmacol. 2016;10:13-18.
- [Google Scholar]
- Effect of aqueous leaf extract of Irvingia gabonensis on the gastrointestinal tract in rodents. Indian J Exp Biol. 2004;42:787-791.
- [Google Scholar]
- Phytochemical observation and antibacterial activity of Cyperus esculentus L. Anc Sci Life. 2009;28:16-20.
- [Google Scholar]
- Evaluation of bioactivity of various Indian medicinal plants: An in vitro study. Int J Intern Med. 2010;8:10.
- [CrossRef] [Google Scholar]
- Mitochondrial dysfunction in psychiatric and neurological diseases: Cause(s), consequence(s), and implications of antioxidant therapy. Biofactors. 2013;39:392-406. doi:10.1002/biof.1093.
- [CrossRef] [PubMed] [Google Scholar]
- Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147-1150. doi:10.1042/BST0351147
- [CrossRef] [PubMed] [Google Scholar]
- Guide for the care and use of laboratory animals (8th ed). The United States: National Academies Press; 2010.
- [Google Scholar]
- The nature of antioxidant defense mechanisms: A lesson from transgenic studies. Environ Health Perspect. 1998;106:1219-1228. doi:10.1289/ehp.98106s51219
- [CrossRef] [PubMed] [Google Scholar]
- Effects of Harungana madagascariensis stem bark extract on the antioxidant markers in alloxan-induced diabetic and carrageenan-induced inflammatory disorders in rats. J Complement Integr Med. 2008;5:2. doi:10.2202/1553-3840.1088
- [CrossRef] [Google Scholar]
- High-throughput determination of malondialdehyde in plant tissues. Anal Biochem. 2005;347:201-207. doi:10.1016/j.ab.2005.09.041
- [CrossRef] [PubMed] [Google Scholar]
- Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopus didymus. Asian Pac Journal Trop Med. 2017;10:792-801. doi:10.1016/j.apjtm.2017.07.024
- [CrossRef] [PubMed] [Google Scholar]
- Phytochemical screening and antioxidant activity of Eryngium creticum. L. Asian Pac J Trop Biomed. 2012;2:1217-1220. doi:10.1016/S2221-1691(12)60388-8
- [CrossRef] [Google Scholar]
- Estimation of malondialdehyde (MDA) by thiobarbituric acid (TBA) assay In: Plant-microbe interactions. New York: Humana; 2021.
- [CrossRef] [Google Scholar]
- Elevated serum MDA and depleted non-enzymatic antioxidants, macro-minerals and trace elements are associated with bipolar disorder. J Trace Elem Med Biol. 2017;39:162-168. doi:10.1016/j.jtemb.2016.09.012
- [CrossRef] [PubMed] [Google Scholar]
- Lipoprotein oxidation and measurement of TBARS formation in a single microliter plate: Its use for the evaluation of antioxidants. Ann Rev Med. 1993;208:10-15. doi:10.1006/abio.1993.1002
- [CrossRef] [PubMed] [Google Scholar]
- Colorimetric assay of catalase. Anal Biochem. 1972;47:389-394. doi:10.1016/0003-2697(72)90132-7
- [CrossRef] [PubMed] [Google Scholar]
- Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515-18517. doi:10.1074/jbc.272.30.18515
- [CrossRef] [PubMed] [Google Scholar]
- Methods for the determination of plasma or tissue glutathione levels. Methods Mol Biol. 2012;889:315-324. doi:10.1007/978-1-61779-867-2_20
- [CrossRef] [PubMed] [Google Scholar]
- GC-MS analysis of Spondias mombin (Linn) methanol leaf extract. Int J Biochem Res Rev. 2021;30:1-7.
- [CrossRef] [Google Scholar]
- Review study on potential activity of Piper betle. J Pharmacogn Phytochem. 2014;3:93-98.
- [Google Scholar]
- Screening and GC-MS profiling of ethanolic extract of Tylophora pauciflora. Bioinformation. 2019;15:425-429. doi:10.6026/97320630015425
- [CrossRef] [PubMed] [Google Scholar]
- Phytochemical, FT-IR, and GC-MS analysis of stem and leaf of Tiliacora acuminata (lan). Hook f and Thomas (menispermaceae) Int J Pharm Sci Res. 2014;5:3977-3986. doi:10.13040/IJPSR.0975-8232.5(9).3977-86
- [CrossRef] [PubMed] [Google Scholar]
- Fatty acids: Health effects of omega-6 polyunsaturated fatty acids In: Encyclopedia of human nutrition (3rd ed). Netherlands: Elsevier; 2013. p. :209-214.
- [CrossRef] [Google Scholar]
- Measuring the antimicrobial activity of lauric acid against various bacteria in human gut microbiota using a new method. Cell Transplant. 2019;28:1528-1541. doi:10.1177/0963689719881366
- [CrossRef] [PubMed] [Google Scholar]
- Chapter 9. Emerging extraction techniques: Microwave-assisted extraction In: Advances in green and sustainable chemistry. Netherlands: Elsevier; 2020. p. :207-224.
- [CrossRef] [PubMed] [Google Scholar]
- Phytochemical and antimicrobial activities of the wild mango Irvingia gabonensis extracts and fractions. Afr J Med Med Sci. 2020;37:119-124.
- [Google Scholar]
- Exogenous salicylic acid ameliorates short-term drought stress in mustard (Brassica juncea L.) seedlings by up regulating the antioxidant defense and glyoxalase system. Aust J Crop Sci. 2013;7:1053-1063.
- [Google Scholar]
- Phytochemical and antimicrobial activities of the wild mango Irvingia gabonensis extracts and fractions. Afr J Med Med Sci. 2020;37:119-124.
- [Google Scholar]
- Free radical scavenging activity and lipoxygenase inhibition of Mahonia aquifolium extract and isoquinoline alkaloids. J Inflamm (Lond). 2007;4:15. doi:10.1186/1476-9255-4-15
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med. 2008;8:63. doi:10.1186/1472-6882-8-63.
- [CrossRef] [PubMed] [Google Scholar]
- Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940-949.
- [CrossRef] [Google Scholar]







