Translate this page into:
Development of emulgel formulation from Markhamia tomentosa leaf extract: Characterization and in vitro antimicrobial activity against skin isolates
*Corresponding author: Bukola Aminat Oseni, PhD Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, University of Lagos, Lagos State, Nigeria. abadebayo@unilag.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Oseni BA, Osekita ST, Ibrahim MB, et al. Development of emulgel formulation from Markhamia tomentosa leaf extract: Characterization and in vitro antimicrobial activity against skin isolates. Am J Pharmacother Pharm Sci 2024:9.
Abstract
Objectives:
Skin infections are mild to severe infections often treated with antimicrobial agents. There is an increase in resistance to antimicrobial agents necessitating the search for new medicines. This study developed an emulgel formulation of Markhamia tomentosa leaf extract and evaluated its antimicrobial activity against skin isolates.
Materials and Methods:
A successive extraction of M. tomentosa leaves in five solvents – n-hexane, dichloromethane, ethyl acetate, ethanol, and distilled water was carried out. The activity of the extracts against skin isolates of Staphylococcus aureus, Staphylococcus epidermidis, and Bacillus subtilis was carried out. The distilled water extract was formulated into emulgel, characterized, and evaluated for antimicrobial activity.
Results:
The n-hexane and dichloromethane extracts of M. tomentosa were inactive against all organisms at concentrations used. The ethanol extract was active against S. aureus and S. epidermidis at 200 mg/mL. The ethyl acetate and distilled water extracts were active against S. epidermidis and B. subtilis at 100 and 50 mg/mL, respectively. All emulgel formulations of distilled water extract of M. tomentosa leaves were brown, smooth, and easy to spread on the skin and had a pH of 4.73–5.55. The formulation containing the distilled water extract was more viscous (7784cP) than the blank formulation (6328cP). The formulation was active against S. epidermidis.
Conclusion:
The distilled water extract of M. tomentosa leaves was the most active against S. epidermidis and B. subtilis. Its emulgel formulation had desirable physicochemical properties for application to the skin. Further studies can be carried out toward its development for the treatment of skin infection caused by organisms investigated.
Keywords
Markhamia tomentosa
Emulgel formulation
Antimicrobial agents
Skin infections
Antimicrobial resistance
INTRODUCTION
Skin and soft-tissue infections (SSTIs) are caused by the microbial invasion of the skin and underlying soft tissues which can result in mild to life-threatening conditions.[1] SSTIs occur in about 24.6/1000 persons annually; about 7–10% of hospitalized patients are diagnosed with SSTIs, representing the third most common diagnosis in the emergency setting after chest pain and asthma.[2] SSTIs are caused by Gram-positive, Gram-negative, or sometimes mixed infections with most infections caused by the Gram-positive organisms – Staphylococcus aureus and Streptococci sp.[3] Antibiotics are administered orally, topically, or intravenously depending on the severity of the infection for the treatment of SSTIs. However, the emergence of resistance among Gram-positive and negative organisms to antibiotics makes the treatment of SSTIs more challenging.[1] This has necessitated the continuous search for new antimicrobial agents.
Medicinal plants represent a source of novel and effective pharmacologically active agents. Over the years, they have been used as a readily available, low-cost alternative to orthodox medicines in the treatment of various ailments including infectious diseases.[4] According to the World Health Organization, over 80% of people in sub-Saharan Africa rely on medicinal plants for their primary health-care needs.[5]
Markhamia tomentosa (Benth.) K. Schum. ex Engl. (Bignoniaceae) is a shrub of about 15 m high found in tropical West Africa and Asia.[6] They are commonly known as Echero in Igbo, Ogie-ikhimwim in Edo, Akoko and Oruru in Yoruba, Nigeria. The plant is used traditionally in the treatment of pain, diarrhea, scrotal elephantiasis, inflammation, and cancer.[7] The leaf extracts of the M. tomentosa plant have been scientifically shown to exhibit antimalarial, antiulcer, antioxidant, anti-inflammatory, analgesic, and antimicrobial activity against some Candida spp., Gram-negative and Gram-positive bacteria.[6,8] M. tomentosa leaf extracts have been shown to contain bioactive compounds, namely, phenolic nuclei, saponins, terpenes, flavonoids, and steroids.[9] Furthermore, two naphthoquinone compounds isolated from the stem bark of M. tomentosa showed antiprotozoal activity against Leishmania donovani, Plasmodium falciparum, and Trypanosoma brucei rhodesiense.[10]
Medicinal plants such as M. tomentosa have gained popularity due to the increasing scientific evidence of their therapeutic activity; however, challenges such as non-solubility, and instability during storage and administration, delivery to non-target sites, affect their efficacy and limit their use.[11] Incorporation of herbal drugs in a suitable drug delivery system enhances solubility, minimizes physical and chemical drug degradation, ensures drug delivery to target sites, protects from toxicity, enhances pharmacological activity, and aids in the sustained delivery of the active pharmaceutical ingredient.[11]
Emulgels are delivery systems that have become popular for the delivery of hydrophobic drugs.[12] It is a drug delivery system with a mixture of emulsion and gel. It has been considered a novel type due to its unique characteristics such as the ability to incorporate either a hydrophilic or hydrophobic drug.[13] Furthermore, the emulgel formulation combines the benefits of emulsion and gels such as controlled and enhanced release of the drug.[13] Emulgels are easily removable, spreadable, greaseless, transparent, non-staining, and emollient, have a long shelf life, esthetically appealing, and hence promote patient acceptability and compliance. They are most widely used for the delivery of many drugs like analgesics, anti-inflammatory, anti-acne, and antifungals.[12]
This study is aimed at developing an emulgel formulation containing M. tomentosa leaf extract and evaluating its antimicrobial activity against skin isolates.
MATERIALS AND METHODS
Materials
The solvents; n-hexane, dichloromethane, ethyl acetate, and ethanol were purchased from CDH, India. Distilled water was obtained from the laboratory. Mueller–Hinton Agar (MHA) was purchased from Oxoid Ltd, UK. Gentamicin injection, Carbopol 934 (Himedia, USA), liquid paraffin, Span 20 was purchased from Fengchen, China. Tween 20 and Tween 80 were purchased from Merck, Germany. Propylene glycol, methylparaben, propylparaben, and triethanolamine (TEA) were obtained from Sigma Aldrich, USA.
Plant collection
Fresh leaves of M. tomentosa (Benth) K. Schum ex Engl. were collected from Oke-Igbo, Ondo state, Nigeria, in January 2023. The leaves were taken to the Herbarium of the Department of Botany, University of Lagos for taxonomic identification and authentication. An herbarium specimen was deposited, and voucher number LUH 9862 was obtained.
Organisms
The skin isolates, namely – Staphylococcus epidermidis, Bacillus subtilis, and S. aureus were provided by the Department of Pharmaceutical Microbiology and Biotechnology, University of Lagos.
Extraction of M. tomentosa leaves
The M. tomentosa leaves were air-dried at room temperature (23°C ± 2°C) for 8 days and blended. A successive extraction of the M. tomentosa leaves was carried out in five solvents, namely, n-hexane, dichloromethane, ethyl acetate, ethanol, and distilled water in their order of increasing polarity. The powdered leaf material (1 kg) was macerated in 2 L of n-hexane for 48 h at room temperature with intermittent shaking. The mixture was filtered and evaporated to dryness using a rotary evaporator (Buchi, Switzerland) at a temperature of 40°C to obtain the n-hexane extract. The marc was air dried at room temperature to completely remove the n-hexane solvent. The dried marc obtained after extraction with n-hexane was macerated in 2.5 L of dichloromethane for 48 h at room temperature. The dichloromethane extract was obtained by filtration and evaporated to dryness using the rotary evaporator. The dry marc was subjected to the maceration process using ethyl acetate, ethanol, and distilled water, respectively. The mixtures were filtered and evaporated to dryness to obtain the extracts.
Antimicrobial activities of the extracts
Test for zone of inhibition
Different concentrations (200, 100, and 50 mg/mL) of the various extracts were prepared using distilled water (for the distilled water and ethanolic extract) or 0.1% Tween 20 in distilled water (for n-hexane, dichloromethane, and ethyl acetate extract) as the solvent. These solvents were used as the negative control while gentamicin (20 µg/mL) was used as the positive control. A culture suspension of the Bacteria (S. epidermidis, B. subtilis, and S. aureus) was prepared and incubated for 3 days. Molten MHA of 25 mL was mixed with 1 mL of each of the organism cultures, transferred into Petri dishes and allowed to solidify. A hole of 12 mm diameter was bore at the center of each quadrant of the Petri dish and 1 mL of the extract was introduced into the hole. The plates were allowed to stand for 4 h and incubated at 37℃ for 24 h. The zones inhibited were measured for each plate.[14]
Determination of minimum inhibitory concentration (MIC)
The MIC for the extracts that showed a zone of inhibition against the organisms tested was determined. A stock concentration of 10.24 mg/mL was prepared for each extract used. A successive 1 in 2 dilution of the stock concentration was carried out to obtain thirteen working concentrations (0.0025–10.24 mg/mL) for each extract. A mixture of the molten agar and the various concentrations of the extract were allowed to solidify, and the organisms were inoculated on each of the plates. The plates were incubated at 37℃ and examined after 24 h. The lowest concentration of the extract where there was inhibition of the growth of the organism was chosen as the MIC.[14]
Preparation of emulgels
Seven emulgel formulations were prepared, as shown in Table 1. The gel portion of the emulgel was prepared by dissolving carbopol-934 in cold water with constant stirring at a moderate speed until a uniform mixture was formed. The pH of the gel was adjusted to 6–6.5 using TEA. For the emulsion component, Tween 80 was dissolved in distilled water to prepare the aqueous phase of the emulsion while for the oil phase of the emulsion, span 20 was dissolved in liquid paraffin. Methylparaben and propylparaben were dissolved in propylene glycol and the extract was dissolved in ethanol. The propylene glycol and extract solutions were mixed with the aqueous phase. The aqueous and the oil phases were heated in a water bath at 70°C separately. The oil phase was added dropwise to the aqueous phase with continuous stirring for 10 min. The emulsion was allowed to cool at room temperature. The gel and emulsion portions were mixed at equal portions stirring moderately and consistently after each addition to avoid air entrapment.[15]
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 |
|---|---|---|---|---|---|---|---|
| Extract (g) | 2.50 | 2.50 | 2.50 | 2.50 | 0.50 | 2.50 | - |
| Carbopol (g) | 0.25 | 0.50 | 0.75 | 0.50 | 0.75 | 0.25 | 0.75 |
| Liquid paraffin (mL) | 3.75 | 3.75 | 3.75 | 2.50 | 2.50 | 2.50 | 2.50 |
| Tween 80 (mL) | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Span 20 (mL) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Propylene glycol (mL) | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 | 3.50 |
| Methylparaben (g) | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Propylparaben (g) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Ethanol (mL) | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Distilled water to (mL) | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
F: Formulation
Characterization of emulgels
Determination of organoleptic properties
The formulations were inspected visually for their color, homogeneity, and consistency. The feel of the emulgels was tested on the skin to check for their smoothness or roughness.
Determination of pH
The pH of each of the emulgels was measured using a Mettler Toledo pH meter (Mettler-Toledo, Switzerland). The electrode was inserted into the formulation and the pH reading was recorded.
Spreadability study
The spreadability test on the emulgel was carried out using a wooden block with a glass slide attached to it and another glass slide with a fixed weight attached to it. On the glass slide that is attached to the wooden block, 1 g of emulgel was placed at the center of the slide in a 2 cm diameter of circle. The second glass slide was carefully placed over it with a fixed weight of 200 g on the slide for 5 min. The increase in diameter of the emulgel was noted.[16]
Determination of viscosity
The viscosity of the emulgel was measured using a Brookfield viscometer (Ametek, USA) with spindle RV-05 at 10, 20, 30, 40, and 50 rpm at 25 ± 0.5℃.
Centrifugation test on emulgels
The stability of the emulgel formulations was determined by centrifugation at 4000 rpm for 10 min. The formulations were examined visually for creaming or phase separation.
Evaluation of the antimicrobial activity of the emulgels
The method of determination of the zone of inhibition for the extracts was used. The molten agar of 25 mL was mixed with 1 mL of each of the susceptible organism cultures (S. epidermidis and B. subtilis), transferred into Petri dishes and allowed to solidify. A hole of 12 mm diameter was bore at the center of each quadrant of the Petri dish using a cork borer and the hole was filled with the emulgel. The plates were allowed to stand for 4 h and the inoculated plates were incubated at 37℃ for 24 h. The zones of inhibition were measured in millimeters using a ruler for each plate. The emulgel without the extract was used as the negative control while gentamicin injection (5%) was used as the positive control.
Statistical analysis
All determinations were carried out in triplicate and the results were expressed as mean ± standard deviation. Statistical difference between the mean values was determined by one-way analysis of variance using the GraphPad® Prism 7 software (GraphPad Software, La Jolla, CA). P < 0.05 was considered significant.
RESULTS
Yield of M. tomentosa extract
The yield of the various extracts obtained from the leaves of M. tomentosa is shown in Table 2. The n-hexane and distilled water extract had the lowest and highest yield, respectively.
| Extract | n-Hexane | Dichloromethane | Ethyl acetate | Ethanol | Distilled water |
|---|---|---|---|---|---|
| Yield (%) | 1.13 | 1.55 | 1.34 | 1.17 | 2.44 |
Evaluation of the antimicrobial activity of M. tomentosa extract
The activities of the five extracts of M. tomentosa, namely, n-hexane, dichloromethane, ethyl acetate, ethanol, and distilled water against three organisms, namely, S. aureus, S. epidermidis, and B. subtilis are presented in Table 3a-e. The n-hexane and dichloromethane extract were inactive against the three organisms at all concentrations employed. The ethyl acetate extract exhibited activity against the three bacteria strains. The ethanol extract showed activity against S. epidermidis and S. aureus while the distilled water extract was active against B. subtilis and S. epidemidis.
| Organisms | Average zone of inhibition (mm) for n-hexane extract (n=3) | ||||
|---|---|---|---|---|---|
| 200 mg/mL | 100 mg/mL | 50 mg/mL | n-hexane | Gentamicin (20 µg/mL) | |
| B. subtilis | - | - | - | - | 26.33±3.79 |
| S. epidermidis | - | - | - | - | 24.33±0.57 |
| S. aureus | - | - | - | - | 23.00±1.00 |
-: No zone of inhibition, B. subtilis: Bacillus subtilis, S. epidermidis: Staphylococcus epidermidis, S. aureus: Staphylococcus aureus, n: number of replicates
| Organisms | Average zone of inhibition (mm) for dichloromethane extract (n=3) | ||||
|---|---|---|---|---|---|
| 200 mg/mL | 100 mg/mL | 50 mg/mL | dichloromethane | Gentamicin (20 µg/mL) | |
| B. subtilis | - | - | - | - | 26.33±3.79 |
| S. epidermidis | - | - | - | - | 24.33±0.57 |
| S. aureus | - | - | - | - | 23.00±1.00 |
-: No zone of inhibition, B. subtilis: Bacillus subtilis, S. epidermidis: Staphylococcus epidermidis, S. aureus: Staphylococcus aureus, n: number of replicates
| Organisms | Average zone of inhibition (mm) for ethyl acetate extract (n=3) | ||||
|---|---|---|---|---|---|
| 200 mg/mL |
100 mg/mL | 50 mg/mL | Ethyl acetate | Gentamicin (20 µg/mL) | |
| B. subtilis | 18.67±0.57 | 16.00±1.00 | - | - | 26.33±3.79 |
| S. epidermidis | 18.33±1.15 | 14.33±0.57 | - | - | 24.33±0.57 |
| S. aureus | 20.00±1.00 | 10.67±0.57 | - | - | 23.00±1.00 |
-: No zone of inhibition, B. subtilis: Bacillus subtilis, S. epidermidis: Staphylococcus epidermidis, S. aureus: Staphylococcus aureus, n: number of replicates
| Organisms | Average zone of inhibition (mm) for ethanolic extract (n=3) | ||||
|---|---|---|---|---|---|
| 200 mg/mL | 100 mg/mL | 50 mg/mL | Ethanol | Gentamicin (20 µg/mL) | |
| B. subtilis | - | - | - | - | 26.33±3.79 |
| S. epidermidis | 20.67±0.57 | 13.33±1.53 | - | - | 24.33±0.57 |
| S. aureus | 13.67±0.57 | - | - | - | 23.00±1.00 |
-: No zone of inhibition, B. subtilis: Bacillus subtilis, S. epidermidis: Staphylococcus epidermidis, S. aureus: Staphylococcus aureus, n: number of replicates
| Organisms | Average zone of inhibition (mm) for distilled water extract (n=3) | ||||
|---|---|---|---|---|---|
| 200 mg/mL | 100 mg/mL | 50 mg/mL | Distilled water | Gentamicin (20 µg/mL) | |
| B. subtilis | - | - | 19.67±0.57 | - | 26.33±3.79 |
| S. epidermidis | - | - | 20.67±0.57 | - | 24.33±0.57 |
| S. aureus | - | - | - | - | 23.00±1.00 |
-: No zone of inhibition, B. subtilis: Bacillus subtilis, S. epidermidis: Staphylococcus epidermidis, S. aureus: Staphylococcus aureus, n: number of replicates
The MICs of ethyl acetate, ethanol, and distilled water extracts that showed antimicrobial activity against some of the organisms tested are represented in Table 4. The distilled water extract was active against B. subtilis and S. epidermidis at the lowest concentration and therefore, more potent than the ethyl acetate and ethanolic extract.
| Organism | MIC (mg/mL) | ||
|---|---|---|---|
| Ethyl acetate | Ethanol | Distilled water | |
| S. aureus | 5.12 | 10.24 | - |
| B. subtilis | 1.28 | - | 0.32 |
| S. epidermidis | 5.12 | 10.24 | 0.64 |
-: No growth inhibition observed, S. aureus: Staphylococcus aureus, B. subtilis: Bacillus subtilis, S. epidermidis: Staphylococcus epidermidis, MIC: Minimum inhibitory concentration
Characterization of emulgels of M. tomentosa extract in distilled water
The emulgel prepared using the distilled water extract of the M. tomentosa leaves is shown in Figure 1. The physicochemical properties and pH of the emulgels are presented in Table 5 and Figure 2, respectively. All emulgel formulations were brown and had a smooth texture with a pH between 4.73 and 5.55.
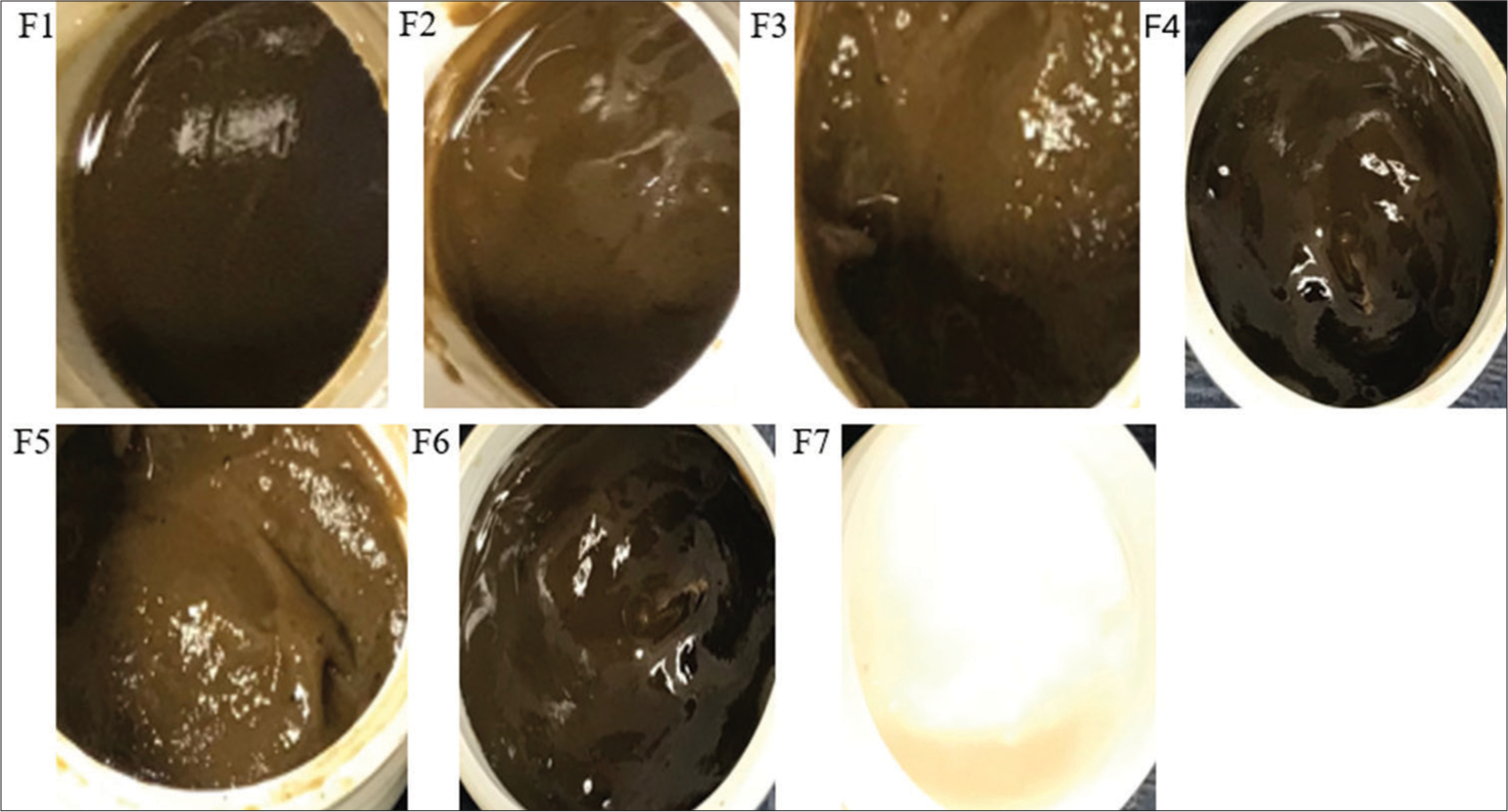
- Emulgel formulation of Markhamia tomentosa extract in distilled water. F1, F2, and F3: 0.5, 1, and 1.5% carbopol, respectively, 7.5% liquid paraffin, 5% extract, F4 and F6: 1 and 0.5% carbopol, respectively, 5% liquid paraffin, 5% extract, F5: 1.5% carbopol, 5% liquid paraffin, 1% extract, F7: 1.5% carbopol, and 5% liquid paraffin. F: Formulation.
| Formulation code | Color | Texture | Spreadability (cm/5 min) |
|---|---|---|---|
| F1 | Light Brown | Smooth | 8.00±0.11 |
| F2 | Light Brown | Smooth | 5.00±0.15 |
| F3 | Light Brown | Smooth | 4.90±0.05 |
| F4 | Dark Brown | Smooth | 5.20±0.25 |
| F5 | Light Brown | Smooth | 5.10±0.11 |
| F6 | Dark Brown | Smooth | 6.90±0.01 |
| F7 | White | Smooth | 5.70±0.13 |
F1, F2 and F3: 0.5, 1 and 1.5% carbopol, respectively, 7.5% liquid paraffin, 5% extract, F4 and F6: 1 and 0.5% carbopol, respectively, 5% liquid paraffin, 5% extract; F5: 1.5% carbopol, 5% liquid paraffin, 1% extract, F7: 1.5% carbopol, 5% liquid paraffins
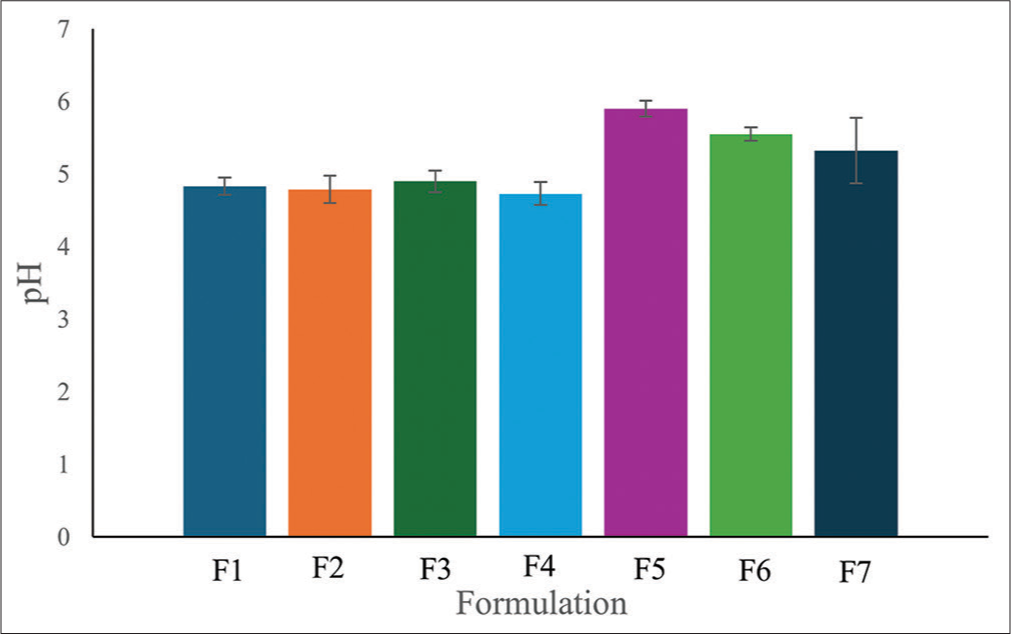
- pH of Markhamia tomentosa emulgels. F1, F2, and F3: 0.5, 1, and 1.5% carbopol, respectively, 7.5% liquid paraffin, 5% extract, F4 and F6: 1 and 0.5% carbopol respectively, 5% liquid paraffin, 5% extract, F5: 1.5% carbopol, 5% liquid paraffin, 1% extract, F7: 1.5% carbopol, and 5% liquid paraffin.
Rheology study on emulgels
The viscosity of the distilled water M. tomentosa extract emulgel formulation with the desirable properties and lower concentration of the extract (F5) and the formulation devoid of the extract (F7) at different speeds is presented in Figure 3. An increase in the speed caused a reduction in the viscosity of both formulations (with or without the extract). The formulation containing the extract is more viscous than the formulation devoid of the extract.
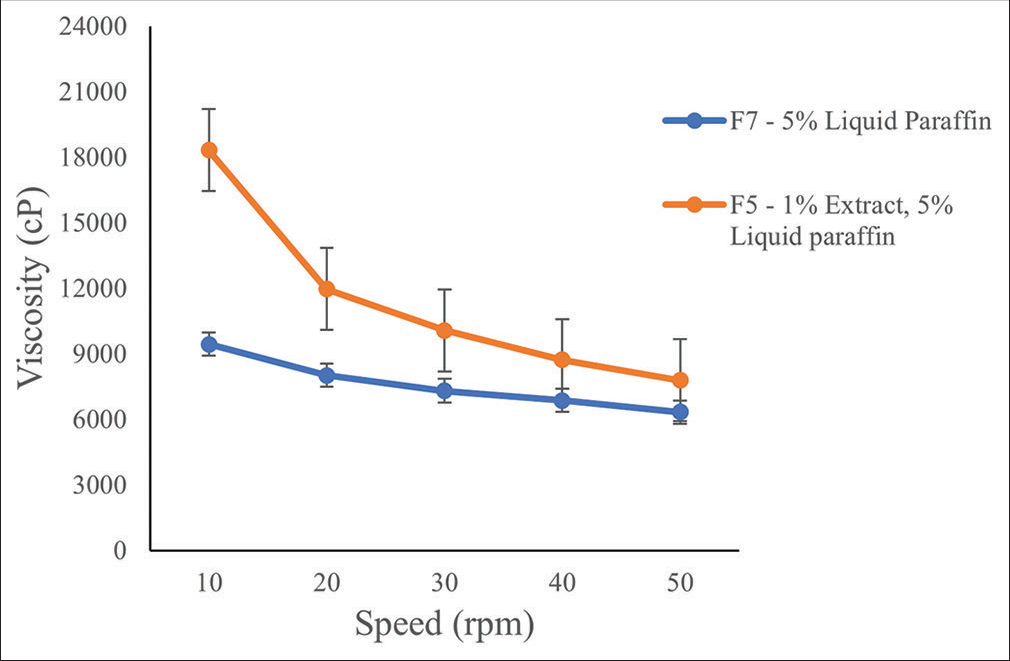
- Viscosity of Markhamia tomentosa emulgel formulation (F) at different speeds.
Stability of emulgels
After the centrifugation test, none of the emulgel formulations showed signs of creaming or phase separation as a form of physical instability.
Evaluation of the antimicrobial activity of emulgel formulation
The zones of inhibition of the formulated emulgel on the active organisms are presented in Table 6. The emulgel formulation was active against S. epidermidis but inactive against B. subtilis.
| Organisms | Zone of inhibition (mm) of emulgel formulation of M. tomentosadistilled water extract | ||||||
|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |
| S. epidermidis | 23.17±0.29 | 19.50±0.50 | 25.17±0.29 | 20.33±0.29 | 23.67±0.58 | 25.33±0.28 | 15.17±0.29 |
| B. subtilis | - | - | - | - | - | - | - |
-: Means no zone of inhibition observed, F1, F2 and F3: 0.5, 1 and 1.5% carbopol, respectively, 7.5% liquid paraffin, 5% extract, F4 and F6: 1 and 0.5% carbopol, respectively, 5% liquid paraffin, 5% extract, F5: 1.5% carbopol, 5% liquid paraffin, 1% extract, F7: 1.5% carbopol, 5% liquid paraffin, F: Formulation.
DISCUSSION
SSTIs are one of the common diagnoses in hospital emergencies often treated with antibiotics; however, the increased incidence of resistance of causative organisms to antibiotics has been a cause for concern and necessitated the exploration of alternative sources of antibiotics. M. tomentosa plant has been shown to possess antimicrobial properties, for example, the ethyl acetate, dichloromethane, and methanol leaves extracts of the plant exhibited antioxidant and antimicrobial activities.[17] The antimicrobial activity of extracts of M. tomentosa against SSTIs-causing bacteria has not been fully explored. In this study, the antimicrobial activity of M. tomentosa leaves extracts against three skin isolates was determined. The extract that was most sensitive to the test micro-organisms was developed into a herbal topical emulgel formulation.
Antimicrobial activity of M. tomentosa extracts
The antimicrobial activity of the five extracts used in this study was evaluated using the zone of inhibition analysis. The zone of inhibition test is a quantitative method used clinically to measure antibiotic resistance and to test the ability of a substance to inhibit microbial growth. This analysis can be used as a measure of the sensitivity of microorganisms to a particular antibiotic and to discover new ones.[18] The n-hexane and dichloromethane extracts had no zone of inhibition on any of the organisms used at 50, 100, and 200 mg/mL concentrations [Table 3a and b]; this shows that they had no antimicrobial activity against the test organisms at the concentrations utilized. The ethyl acetate extract [Table 3c] was active against all bacteria tested while the ethanolic extract [Table 3d] was active against the Staphylococcus spp. tested. This is in line with the findings of Aladesanmi et al., 2007.[19]
The distilled water extract was active against S. epidermidis and B. subtilis at 50 mg/mL concentration [Table 3e], higher concentrations showed no activity against the organisms. In most cases, a higher concentration of a medicinal agent causes a higher therapeutic activity, in this case, activity was observed at a lower concentration. This may be because the bioavailability of the extract is dependent on its concentration and pH.[20] The standard antimicrobial agent – gentamicin was utilized in this study because it has broad activity against many Gram-negative organism and when combined with other antibiotics, exhibits antimicrobial activity against some Gram-positive organisms such as S. aureus.[21] Gentamicin exhibited antimicrobial activity against the bacteria tested at concentrations as low as 0.02 mg/mL.
According to Eucast definitive Document Methods for the determination of susceptibility of bacteria to antibiotics, MIC is the lowest concentration of antimicrobial drug that will inhibit the visible growth of a microorganism after incubating overnight at 37℃.[22] The MIC is a measure of the sensitivity of the medicinal agent to the test organism, the lower the MIC, and the more sensitive the medicinal agent. The ethyl acetate extract of M. tomentosa leaves was active against S. epidermidis, S. aureus, and B. subtilis; however, it is more sensitive to B. subtilis having a MIC of 1.28 mg/mL than the Staphylococcus spp. tested whose MIC was 5.12 mg/mL [Table 4]. The ethanolic extract was active against only S. epidermidis and S. aureus having similar potency with an MIC of 10.24 mg/mL [Table 4]. The distilled water extract exhibited the highest sensitivity to S. epidermidis (MIC of 0.64 mg/mL) and B. subtilis (MIC of 0.32 mg/mL) [Table 4]. Therefore, the distilled water extract was chosen for formulation into emulgel.
Physicochemical evaluation of M. tomentosa emulgels
The emulgel formulations of M. tomentosa distilled water extract were assessed for their organoleptic properties. The color of the formulations ranges from light brown (F1, F2, F3, and F5) to dark brown (F4 and F6) [Table 5]. The color observed was due to the M. tomentosa introduced into the formulation. The dark brown color observed in F4 and F6, may be due to the reduction in the quantity of liquid paraffin and carbopol in these two formulations; formulation F7 appeared white due to the absence of extract in the formulation. All the emulgels had a smooth feel on the skin and were easily washable after application. The pH of the emulgels falls within 4.73–5.55 [Figure 2]. In general, topical formulations are developed to have a similar pH as the intact human skin of 4–6 to prevent skin irritation and maintain a healthy skin environment.[23] All emulgels fall within the specified range; hence, the formulations can be said to be suitable for application to the skin.
The spreadability of a topical formulation is an important property in the development of an ideal formulation. It is used to determine the extent to which a formulation will spread on application to the skin to enhance skin penetration and bioavailability of the active ingredient. Formulations with high spreading value depict better spreadability and skin penetration ability.[24] All emulgel formulations were easily spreadable but F1 and F6 were very spreadable. This can be attributed to a reduction in the concentration of the thickener and gelling agent – Carbopol (0.5%) in both formulations when compared with the other formulations (1 or 1.5% Carbopol).
The resistance of a fluid to deforming under shear force is measured by its viscosity. It is frequently interpreted as flow behavior or pouring resistance. Viscosity is described as a fluid’s internal resistance to flow. An increase in the viscosity of a formulation together with other factors increases its stability.[25] An increase in viscosity was observed in the emulgel formulation with the extract (F5) when compared with the emulgel without the extract (F7) [Figure 3]. Therefore, the extract causes an increase in the viscosity of the formulation. Furthermore, a decrease in the viscosity was observed in both formulations following an increase in the shear stress from 10 rpm to 50 rpm. This is an attribute of a non-Newtonian shear-thinning fluid whose viscosity decreases with increased stress.[26] Therefore, the amount of shear to be used during the preparation of the emulgel should be optimized to produce a stable product with desired fluidity for easy application. In addition, the viscosity of a gel is inversely proportional to its spreadability. An increase in spreadability will infer a low viscosity of the gel or product. This was observed in the F5 and F7 formulations, the latter was more spreadable [Table 5] and less viscous [Figure 3] than the former. Higher viscosity promotes stability of the gel; however, this can cause poor spreadability and poor skin penetration of the formulation; therefore, there is a need for a balance in viscosity and spreadability to obtain an ideal product.[27]
Antimicrobial evaluation of emulgel
Antimicrobial screening was carried out on all the formulations to check if the extracts would retain their antimicrobial activity after formulating as an emulgel. All six formulations inhibited the growth of S. epidermidis but not B. subtilis. Formulations F1, F3, F5, and F6 had a higher zone of inhibition than the extract alone while the zone of inhibition of F2 and F4 is the same as the extract for S. epidermidis [Table 6]. The formulation containing 1% extract (F5) had similar zones of inhibition to those containing 5% extract (F1, F3, and F6). The formulation without the extract also showed the least zones of inhibition against S. epidermidis which suggests an inherent activity. All formulations showed no activity against B. subtilis whereas the extract was active against the organism. This may be attributed to the interaction between the extract and the excipients which might have hindered the activity of the formulation against the test organism. A systematic change in the excipient and the utilization of different excipients might retain the activity of the extract in the formulation.
CONCLUSION
The ethyl acetate, ethanol, and distilled water extracts of M. tomentosa leaves were active against S. aureus, S. epidermidis, and B. subtilis. The distilled water extract was the most susceptible to S. epidermidis and B. subtilis, hence used in the formulation of the emulgel. The emulgel prepared was light to dark brown, of smooth feel, easy to spread, suitable for application to the skin, and active against S. epidermidis. Therefore, an herbal topical formulation of M. tomentosa extract was developed for the treatment of skin infections caused by S. epidermidis. Further characterizations and studies need to be done to ascertain its suitability for development as a topical antibiotic.
Acknowledgment
We thank Mr. Abdulrahman Usman of the Department of Pharmaceutical Microbiology and Biotechnology, University of Lagos for providing the microbial organisms utilized in this study.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
None.
References
- Current epidemiology, etiology, and burden of acute skin infections. Clin Infect Dis. 2019;68:S193-S199. Doi: 10.1093/cid/ciz002
- [CrossRef] [PubMed] [Google Scholar]
- Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008;19:173-184. Doi: 10.1155/2008/846453
- [CrossRef] [PubMed] [Google Scholar]
- Epidemiology and microbiology of skin and soft tissue infections. Curr Opin Infect Dis. 2016;29:109-115. Doi: 10.1097/QCO.0000000000000239
- [CrossRef] [PubMed] [Google Scholar]
- Phytochemical screening and antimicrobial activity evaluation of selected medicinal plants in Ethiopia. J Exp Pharmacol. 2023;15:51-62. Doi: 10.2147/JEP.S379805
- [CrossRef] [PubMed] [Google Scholar]
- Traditional medicine strategy 2014-2023. 2013. Geneva: World Health Organization; :1-76. Available from: https://www.who.int/publications/i/item/9789241506096 [Last accessed on 2024 Feb 15]
- [Google Scholar]
- Review of the phytochemical and pharmacological studies of the genus Markhamia. Pharmacogn Rev. 2016;10:50-59. Doi: 10.4103/0973-7847.176547
- [CrossRef] [PubMed] [Google Scholar]
- Antifungal and antiproliferative activities of endophytic fungi isolated from the leaves of Markhamia tomentosa. Pharm Biol. 2017;55:590-595. Doi: 10.1080/13880209.2016.1263671
- [CrossRef] [PubMed] [Google Scholar]
- Phytochemical screening and in vivo antimalarial activity of extracts from three medicinal plants used in malaria treatment in Nigeria. Parasitol Res. 2015;115:299-305. Doi: 10.1007/s00436-015-4747-x
- [CrossRef] [PubMed] [Google Scholar]
- Phytochemical and ethnobotanical study of some selected medicinal plants from Nigeria. J Med Plant Res. 2012;6:1106-1118. Doi: 10.5897/JMPR09.430
- [CrossRef] [Google Scholar]
- Activities of some Nigeria medicinal plants against Aedes aegypti. Chin Med. 2012;3:151-156. Doi: 10.4236/cm.2012
- [CrossRef] [Google Scholar]
- Novel herbal drug delivery system: An overview. Arch Med Health Sci. 2018;6:171-179. Doi: 10.4103/amhs.amhs_88_17
- [CrossRef] [Google Scholar]
- Emulgel; an effective drug delivery system. Drug Dev Ind Pharm. 2021;47:1193-1199. Doi: 10.1080/03639045.2021.1993889
- [CrossRef] [PubMed] [Google Scholar]
- Emulgel: Magnifying the application of topical drug delivery. Indian J Pharm Biol Res. 2017;5:25-33. Doi: 10.30750/ijpbr.5.1.4
- [CrossRef] [Google Scholar]
- Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6:71-79. Doi: 10.1016/j.jpha.2015.11.005
- [CrossRef] [PubMed] [Google Scholar]
- Development of antifungal emulsion based gel for topical fungal infection. Int J Pharm Res Dev. 2011;2:18-25.
- [Google Scholar]
- Mucoadhesive micro emulsion based prolonged release vaginal gel for anti-fungal Drug. Am J Pharm Tech Res. 2012;2:650-661.
- [Google Scholar]
- Protective activity of Markhamia tomentosa (Benth.) K. Schum. (Bignoniaceae) methanol leaves extract against galactosamine/lipopo-lysaccharide-induced acute liver injury in mice. J Biosci Med. 2020;8:74-89. Doi: 10.4236/jbm.2020.810008
- [CrossRef] [Google Scholar]
- The zone of inhibition. Clin Chem. 2009;65:819. Doi: 10.1373/clinchem.2018.299800
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial and antioxidant activities of some Nigerian medicinal plants. Afr J Tradit Complement Altern Med. 2006;4:173-184. Doi: 10.4314/ajtcam.v4i2.31206
- [CrossRef] [PubMed] [Google Scholar]
- Effects of pH on antioxidant and antimicrobial properties of tea saponins. Eur Food Res Technol. 2009;228:1023-1028. Doi: 10.1007/s00217-009-1014-3
- [CrossRef] [Google Scholar]
- Gentamicin In: LiverTox: Clinical and research information on drug-induced liver injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548706 [Last accessed on 2024 Apr 17]
- [Google Scholar]
- EUCAST Definitive Document: Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin Microbiol Infect. 2000;6:503-8.
- [Google Scholar]
- Development of diclofenac and capsaicin emulgel for the management of inflammation in rheumatoid arthritis. Trop J Nat Prod Res. 2023;7:3246-3252.
- [CrossRef] [Google Scholar]
- Formulation of micro emulsion gel systems for transdermal delivery of celecoxib: In vitro permeation, anti-inflammatory activity and skin irritation tests. Drug Disc Ther. 2010;4:459-471.
- [Google Scholar]
- An overview of non-Newtonian fluid. Int J Appl Sci Eng. 2016;4:97-101. Doi: 10.5958/2322-0405.2016.00011.3
- [CrossRef] [Google Scholar]
- Physical chemistry evaluation of stability, in vitro antioxidant and photoprotective capacities of topical formulation containing Calendula officinale L leaf extract. Braz J Pharm Sci. 2015;5:63-75. Doi: 10.1080/03639045.2021.1993889
- [CrossRef] [Google Scholar]







