Translate this page into:
Fulminant Neisseria septicemia with secondary hemophagocytic lymphohistiocytosis
*Corresponding author: Harish Kasarabada, Department of Internal Medicine, Army Hospital Research and Referral, Delhi, India. kasarabadaharish@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kasarabada H, Akhtar Z, Devaraju U. Fulminant Neisseria septicemia with secondary hemophagocytic lymphohistiocytosis. Am J Pharmacother Pharm Sci 2025:007.
Abstract
A 26-year-old male soldier stationed in hilly terrain presented with a febrile illness followed by migratory inflammatory arthritis and non-blanchable petechial lesions over the lower limbs. On admission to a tertiary center, he exhibited respiratory distress, a pansystolic murmur, and elevated acute-phase reactants. Initial investigations suggested sepsis with suspicion of infective endocarditis, but transthoracic echocardiography was unremarkable. Bone marrow examination revealed hemophagocytic lymphohistiocytosis (HLH), and bone marrow cultures identified Neisseria meningitidis, confirming fulminant meningococcal septicemia as the underlying cause. The patient was treated with intravenous immunoglobulin and broad-spectrum antibiotics, resulting in significant clinical improvement. This case highlights the rare presentation of N. meningitidis septicemia complicated by secondary HLH and the importance of early diagnosis and multidisciplinary management.
Keywords
Hemophagocytic lymphohistiocytosis
Infective endocarditis
Purpura fulminans
Septicemia
INTRODUCTION
Neisseria meningitidis is a Gram-negative encapsulated diplococcus responsible for invasive meningococcal disease, which manifests as meningitis or septicemia. Globally, it accounts for approximately 1.2 million cases annually, with a mortality rate of up to 15% despite treatment.[1] Fulminant meningococcal septicemia is a severe form characterized by disseminated intravascular coagulation, multi-organ dysfunction syndrome, and purpuric rash.[2] Secondary hemophagocytic lymphohistiocytosis (HLH) is a rare hyperinflammatory syndrome triggered by infections, with N. meningitidis rarely implicated. HLH is marked by excessive activation of immune cells leading to cytokine storm, hemophagocytosis, and multi-organ failure. Early recognition and treatment are critical, as mortality exceeds 50% if left untreated.[3] This report discusses a unique case of fulminant N. meningitidis septicemia complicated by HLH in a young soldier, emphasizing diagnostic challenges and therapeutic strategies.
CASE REPORT
Clinical history
A 26-year-old male soldier stationed in a remote hilly region presented with:
Day 1–2: High-grade fever
Day 3–4: Migratory inflammatory arthritis involving large joints
Day 5: Non-blanchable petechial lesions over the lower limbs.
He subsequently developed worsening fatigue and dyspnea on exertion. On arrival at the tertiary center, he reported progressive respiratory distress. His significant examination findings at the time of presentation included:
Respiratory System: Increased work of breathing, bilateral fine crackles in the infrascapular areas
Cardiovascular System: Grade 3 pansystolic murmur in the mitral area
Dermatologic Findings: Non-blanchable petechial rash on the lower limbs [Figure 1] that generalized over time with Janeway lesions on the palms [Figure 2].
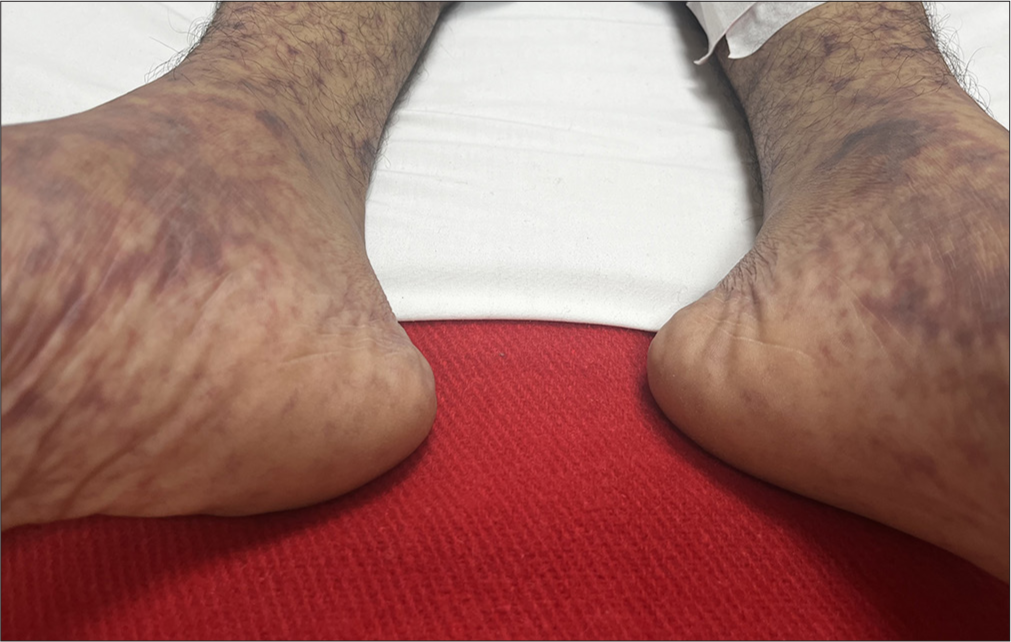
- Non-blanchable petechial rash on the lower limbs.
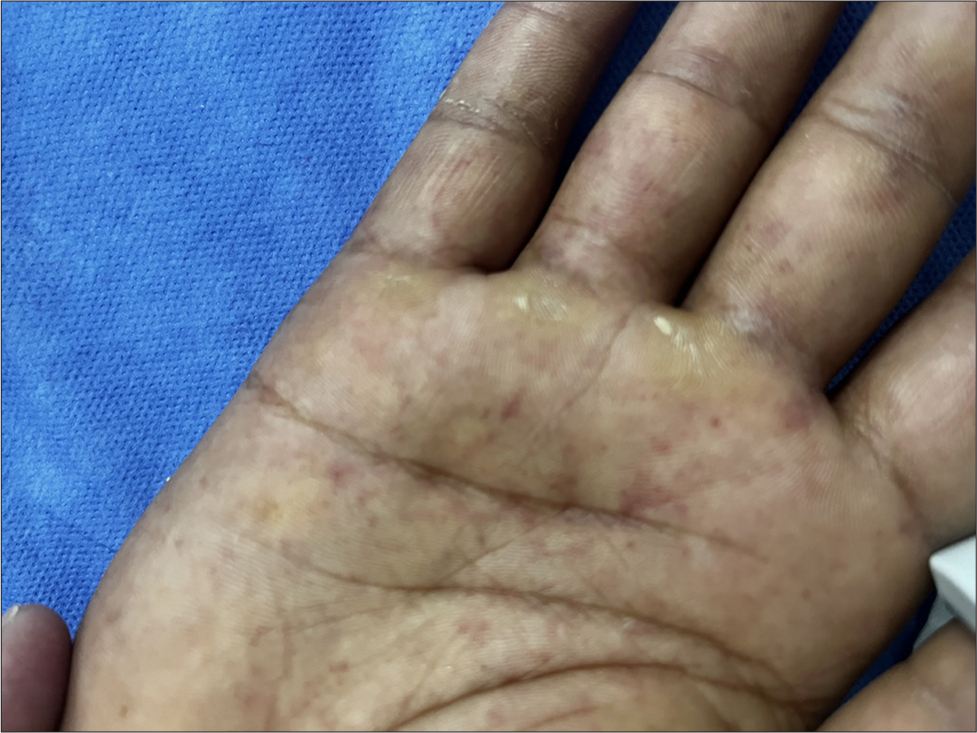
- Janeway lesions on the palms.
Laboratory parameters showed anemia, polymorphic leukocytosis, thrombocytopenia, elevated alanine aminotransferase and aspartate transaminase (>3× upper limit of normal), hyperferritinemia (2,100 ng/mL), and high prolactin and hypertriglyceridemia (345 mg/dL). A bacterial septicemia diagnosis was made and was started on broad-spectrum antibiotics (ceftriaxone, vancomycin, doxycycline) along with oxygen support. Blood and urine cultures were collected before initiating antibiotics; however, the patient reported taking amoxicillin-clavulanate for 2 days before arrival, which may have influenced culture results. In view of elevated ferritin levels and triglyceride levels, splenomegaly and with a normal coagulation profile, HLH was suspected. Bone marrow aspiration histology showed evidence of hemophagocytes, confirming the diagnosis of HLH [Figure 3] as per HLH-2004 criteria. Transthoracic echocardiography (TTE) revealed moderate mitral regurgitation, however, had no vegetation in any of the valves; however, the absence of vegetation does not rule out the diagnosis of IE. Chest imaging indicated bilateral pulmonary edema secondary to acute mitral regurgitation (MR). Although blood and urine cultures were sterile, bone marrow culture confirmed the presence of N. meningitidis [Figure 4]. Intravenous immunoglobulin (IVIG) at dosing 2 grams/kilogram body weight divided into 5 days was started along with dexamethasone and was continued on culture-sensitive antibiotics. Oxygen requirements reduced drastically within 48 hours, pulmonary edema secondary to acute left ventricle failure due to MR resolved, and the patient became afebrile by day 3 of IVIG therapy. Rash regressed, and organ function normalized. The patient was discharged in a stable condition after 10 days, with complete resolution of symptoms, and steroids were slowly tapered on subsequent follow-up.
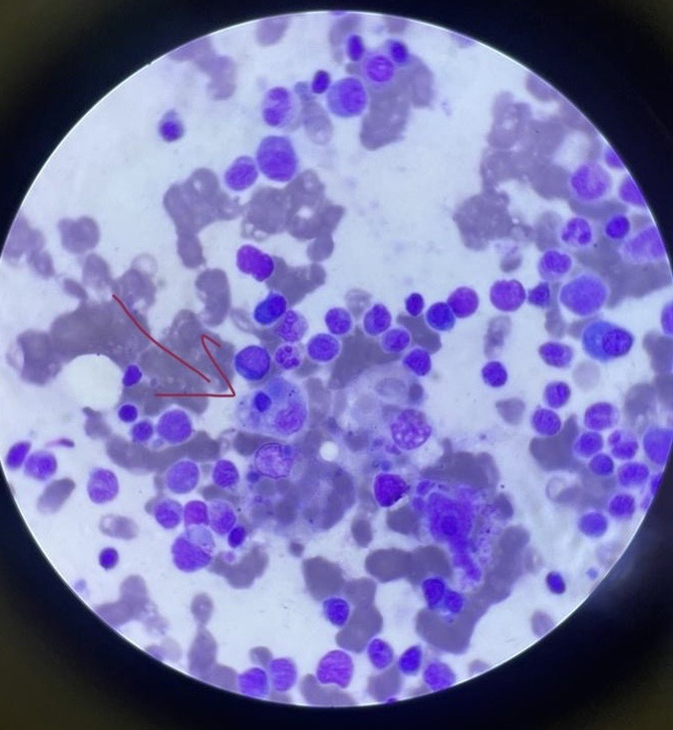
- Bone marrow aspiration histology showed evidence of hemophagocytes (red arrow).
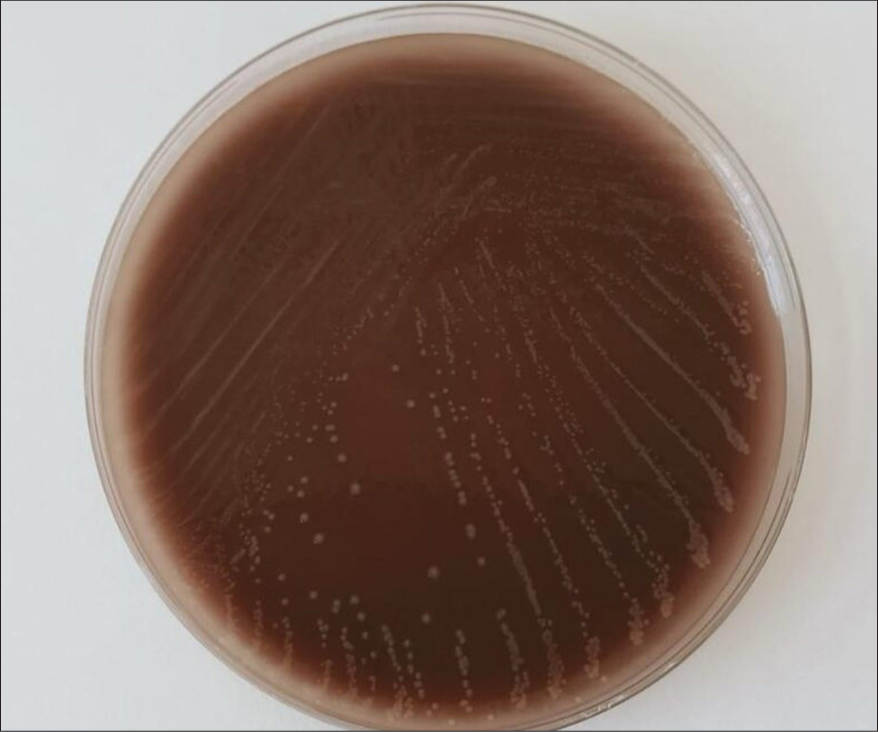
- Bone marrow culture showed growth of Neisseria meningitidis on chocolate agar medium.
DISCUSSION
While fulminant N. meningitidis septicemia is a recognized clinical entity, its progression to secondary HLH is very rare. Most cases of HLH are triggered by viral infections, with bacterial causes accounting for <10% of cases.[4] Invasive N. meningitidis infections typically present as meningitis or septicemia, with skin manifestations such as petechial or purpuric rash in 50–75% of cases.[5] The addition of HLH and vasculitic lesions, as observed here, represents an unusual and severe presentation. This case highlights the challenges in early identification of sepsis and its progression to secondary HLH. Bone marrow biopsy played a crucial role in confirming HLH. Moreover, sterile blood cultures due to prior antibiotic therapy necessitated reliance on bone marrow culture for identifying N. meningitidis.[6] Secondary HLH results from excessive immune activation triggered by infections. The hallmark is hemophagocytosis, driven by the release of cytokines such as tumor necrosis factor-alpha and interleukin-6, which perpetuate the hyperinflammatory state.[7] In this case, the endotoxin-mediated systemic inflammation of N. meningitidis likely initiated the HLH cascade. The global incidence of invasive meningococcal disease is estimated at 1.2 million cases annually, with a mortality rate of 10–15% despite treatment. Vaccination has reduced the disease burden, but vaccine escape strains remain a concern.[8] The prognosis of meningococcal septicemia improved significantly with early administration of antibiotics and early immunomodulatory therapy in HLH reduces mortality by 50%.[9] In this patient, rickettsial illness was suspected initially with a history of fever, cytopenias, and a history of living in hilly terrain areas, in view of the same doxycycline was started as part of broad-spectrum empirical antibiotic therapy. Acute left ventricular failure was secondary to MR, MR in this case could be secondary to septicemia leading to myocardial dysfunction and subsequently valvular dysfunction[10] or due to infective endocarditis (IE) which was not visible on TTE. IE was suspected in view of the new development of cardiac murmur along with Janeway lesions which is a characteristic of septic emboli; however, demonstration of vegetation on valves is key for diagnosing the same along with positive blood culture. The absence of vegetation on TTE cannot totally rule out the diagnosis of IE, transesophageal echocardiography (TEE) is required in highly suspicious cases.[11] Due to the unavailability of TEE, despite sterile serial blood cultures and no vegetation on TTE in view of high clinical suspicion of IE (septic emboli and MR) empirical antibiotics (Ceftriaxone and Vancomycin) were started in this patient. HLH was diagnosed as per HLH 2004 protocol guidelines. IVIg being the standard care of treatment, steroids were also started in this case in view of rapid progression to multiorgan involvement.[12] The combination therapy along with early identification of HLH led to a remarkable recovery in this case.
Learning points
Clinicians should consider secondary HLH in patients with severe sepsis and unexplained hyperinflammation. Bone marrow aspiration should be performed when blood cultures are sterile, especially in cases with suspected bacterial sepsis and persistent inflammation.
CONCLUSION
This case underscores the critical importance of recognizing atypical complications of common infections. Fulminant N. meningitidis septicemia with HLH represents a life-threatening but treatable condition when diagnosed promptly. Clinicians should maintain a high index of suspicion for HLH in cases of severe sepsis with unexplained hyperinflammation, and bone marrow evaluation should be considered in ambiguous cases.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The author confirms that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship: None.
References
- Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267-1284.
- [CrossRef] [PubMed] [Google Scholar]
- Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev. 2000;13(1):144-166.
- [CrossRef] [PubMed] [Google Scholar]
- Pathogenesis of hemophagocytic lymphohistiocytosis. Hematol Oncol Clin N Am. 2015;29:895-902.
- [CrossRef] [PubMed] [Google Scholar]
- Approach to hemophagocytic syndromes. Hematology. 2011;2011(1):178-183.
- [CrossRef] [PubMed] [Google Scholar]
- Fatal meningococcal septicemia without meningeal signs, contribution of the peripheral smear in diagnosis: Report of a case. Indian J Pathol Microbiol. 2018;61(2):284.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat Rev Nephrol. 2019;15(7):435-455.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic challenges of hemophagocytic lymphohistiocytosis. Clin Lymphoma Myeloma Leuk. 2017;17:S105-110.
- [CrossRef] [PubMed] [Google Scholar]
- Impact of meningococcal B vaccine on invasive meningococcal disease in adolescents. Clin Infect Dis. 2021;73(1):e233-237.
- [CrossRef] [PubMed] [Google Scholar]
- An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+T cells and interferon gamma are essential for the disorder. Blood. 2004;104(3):735-743.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes and risk factors of septic shock in patients with infective endocarditis: A prospective cohort study. Open Forum Infect Dis. 2021;8(6):ofab119.
- [CrossRef] [PubMed] [Google Scholar]
- Transthoracic echocardiography (TTE): Sufficiently sensitive screening test for native valve infective endocarditis (IE) Heart Lung. 2011;40(4):358-360.
- [CrossRef] [PubMed] [Google Scholar]
- Efficient management of secondary haemophagocytic lymphohistiocytosis with intravenous steroids and g-immunoglobulin infusions. World J Clin Cases. 2019;7(21):3394-3406.
- [CrossRef] [PubMed] [Google Scholar]






