Translate this page into:
Pharmacological management of upper respiratory tract infections in children: An assessment of a tertiary institution practice in Nigeria
*Corresponding author: Patricia U. Ogbo, PhD Department of Clinical Pharmacy and Biopharmacy, Faculty of Pharmacy, University of Lagos, Nigeria. pogbo@unilag.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Ogbo PU, Obeka IC, Ayeni FA, et al. Pharmacological management of upper respiratory tract infections in children: An assessment of a tertiary institution practice in Nigeria. Am J Pharmacother Pharm Sci 2023;013.
Abstract
Objectives:
Upper respiratory tract infections (URTIs) are the most common acute RTIs that occur in children. Therapy addressing symptoms is recommended for URTI management. The use of antibiotics without culture and sensitivity tests is a risk factor for antimicrobial resistance.
Materials and Methods:
This study was set to assess the pharmacological management of URTIs in children from a tertiary institution practice. This study was conducted in Alex Ekwueme Federal University Teaching Hospital, Abakaliki Ebonyi State, Nigeria. It was a retrospective review of 275 prescriptions of medicines for URTIs in children, from January to December 2021. A systematic sampling technique was used to collect data. Data were analyzed using the Statistical Package for the Social Sciences version 28.0.
Results:
Of the 275 sampled URTI cases, 157 (57.1%) occurred in male children. No laboratory test was conducted for 265 (96.4%) cases. The majority (207; 75.3%) were diagnosed as non-specific URTI. Other diagnoses include tonsillitis (46; 16.7%), otitis media (8; 2.9%), and rhinitis (6; 2.2%) among others. Antibiotics were the most prescribed medications, (212; 77.1%) followed by antihistamines, (110; 40.0%), vitamins (75; 27.3%), and antimalarials (55; 20.0%). The most prescribed antibiotic was amoxicillin-clavulanic acid (79; 28.7%). There was no statistically significant association between the age category of children and the number of medicines prescribed, whether antibiotics were prescribed, and the number of antibiotics prescribed (P > 0.05).
Conclusion:
Antibiotics were the mainstay for the management of URTIs in the study center. This mode of therapy could lead to antimicrobial resistance since culture and sensitivity tests were not done before the initiation of antibiotic therapy.
Keywords
Antibiotics
Antimicrobial resistance
Children
Upper respiratory tract infection
INTRODUCTION
Respiratory diseases are a leading cause of morbidity and mortality in the world as they impose an immense global health burden.[1] Acute respiratory tract infections (RTIs) comprise 50.4–60.8% of all respiratory illnesses among children under 5 years of age.[2-4] It is a major reason for hospital visits in this age group, while pneumonia accounts for 20% of RTIs and is a leading cause of mortality.[3,5-7] Multiple factors such as the host factors, environmental factors, and infecting agents determine the nature and frequency of occurrence of RTIs. Scholars have found specific factors that contribute to the occurrence of RTIs: The presence of the child in the kitchen while cooking, religion followed by the family and presence of RTI in the family, living in rural areas, parental smoking, overcrowding, place of residence, and mother’s education.[2-4] Majority of RTIs are upper RTIs, mainly of viral origin, and self-limiting. Upper RTI is anatomically described as an infection of the upper airways from the nostrils to the vocal cords in the larynx including the paranasal sinuses and the middle ear without signs of pneumonia.[6] Hence, therapy addressing symptoms is recommended for most upper RTIs (URTIs) as they are self-limiting viral infections that resolve without prescription drugs in most cases.[8,9] A study conducted in Port-Harcourt, south-south Nigeria found URTIs presenting as cough, catarrh, and tonsillitis.[10] Although the mainstay of treatment is symptomatic management, studies have shown that 50–70% of total antibiotic prescriptions are attributed to URTIs even though most cases are of viral origin.[11-14] The use and overuse of antibiotic therapy in the management of URTIs have become an important clinical problem, as it increases the risks and rates of microbial resistance.[15-17] A study conducted among 403 mothers in Enugu, southeastern Nigeria showed that 95.3% of them opted for treatment of their children’s suspected URTI with unprescribed antibiotics.[18]
Tertiary hospitals are expected to provide the highest level of healthcare including optimal pharmacological management of diseases. However, pharmacotherapeutic data for the management of URTI in tertiary institutions in Nigeria are sparse. This study was designed to assess the pharmacological management of URTIs in children in a tertiary institution in Nigeria and the findings will be resourceful for other tertiary institutions.
MATERIALS AND METHODS
Study settings
The study was conducted in the Children’s Out-patients Clinic of Alex Ekwueme Federal University Teaching Hospital, Abakaliki (AE-FUTHA) in southeastern Nigeria.
Study design
The study was a descriptive retrospective review of prescribing records for URTIs encountered from January 2021 to December 2021. The 12-month period was utilized to accommodate seasonal influences.
Study population
The study population included case folders of children 5 years old and younger who were diagnosed with URTIs. Case folders were identified from the out-patient register with their identity numbers. All cases of URTI that accessed care in the weeks under review were included in the study. Cases of URTIs with comorbidities such as malaria, diarrhea, and other disease conditions were excluded from the study.
Sampling technique
The systematic sampling method, a fixed periodic interval, was used to select the case folders. The first 5 clinic days at the beginning of each month of the 12 months in the study period were randomly chosen (60 days). This was done to ensure that every prescriber had their prescription represented, because the prescribers in the children’s outpatient department consulted on different days of the week.
Data collection
A standardized data collection pro forma, which had been used in similar studies was adopted for this study.[19] Information was extracted from children’s outpatient clinic registers where all prescribing details were recorded. Prescriptions with diagnoses of non-specific URTIs, and presentations of URTIs such as tonsillitis, pharyngitis, catarrh, rhinitis, common cold, sore throat, rhinosinusitis, cough, or otitis media were collated for assessment.[20] Information collected include date of prescription, gender of child, age of child (in months), complaints, diagnoses, investigations done, and medicines prescribed as indicated in the registers. Folders of children with comorbidities were excluded from the study.
Data analysis
Collected data were coded into Statistical Package for the Social Sciences Version 28.0 and, then, analyzed. Results were presented as proportions (percentages) and frequencies using frequency tables and bar charts. Pearson’s correlation coefficient was used to find association between quantitative variables. A comprehensive guideline for the management of RTIs as provided by the Nigerian Standard treatment guidelines was used to assess the medications prescribed.[8]
Ethical approval
Ethical approval was obtained from the Health Research and Ethics Committee of AE-FUTHA (AE-FUTHA/REC/VOL 3/2022/008).
RESULTS
Sociodemographic characteristics
A total of 275 case notes were assessed for this study. Ninety patients (32.7%) were between 1 and 11 months of age while 17 (6.2%) were 60 months of age. Only one patient, (0.4%) was <1 month old. Mean age was 23.29 ± 17.287 (months). Gender wise, 157 (57.1%) of the cases were for males while females accounted for 118 (42.9%) [Table 1].
| Variable | Characteristics | Frequency (%) |
|---|---|---|
| Age (months) | <1 | 1 (0.4) |
| Mean age=23.29±17.287 | 1-11 | 90 (32.7) |
| 12-23 | 56 (20.4) | |
| 24-35 | 44 (16.0) | |
| 36-47 | 42 (15.3) | |
| 48-59 | 25 (9.1) | |
| 60 | 17 (6.2) | |
| Total | 275 (100) | |
| Gender | Male | 157 (57.1) |
| Female | 118 (42.9) | |
| Total | 275 (100) |
Laboratory tests and diagnoses
Almost all the patients (265; 96.4%) did not carry out any laboratory tests. The diagnoses included non-specific URTIs (207; 75.3%), tonsillitis (46; 16.7%), and otitis media (8; 2.9%) among others [Table 2].
| Variables | Characteristics | Frequency (%) |
|---|---|---|
| Tests | None | 265 (96.4) |
| Full blood count | 1 (0.4) | |
| Malaria Rapid Diagnostic | 1 (0.4) | |
| Test, Microscopic Culture and | ||
| Sensitivity, throat swab | ||
| Full blood count, Malaria | 1 (0.4) | |
| parasite, blood film | ||
| Ear Swab | 1 (0.4) | |
| Malaria Rapid Diagnostic Test | 1 (0.4) | |
| Full blood count, blood film | 1 (0.4) | |
| Xray, blood film, nasal fixture | 1 (0.4) | |
| Malaria parasite, blood group | 1 (0.4) | |
| Fasting blood sugar, stool test | 1 (0.4) | |
| Full blood count, blood film, | 1 (0.4) | |
| Microscopic Culture and | ||
| Sensitivity, throat swab | ||
| Total | 275 (100) | |
| Diagnoses | Non-specific URTI | 207 (75.3) |
| Tonsillitis | 46 (16.7) | |
| Otitis media | 8 (2.9) | |
| Rhinitis | 6 (2.2) | |
| Cough | 5 (1.8) | |
| Common cold | 1 (0.4) | |
| Cough/common cold/URTI | 1 (0.4) | |
| Rhinosinusitis | 1 (0.4) | |
| Total | 275 (100) |
Prescription of medications
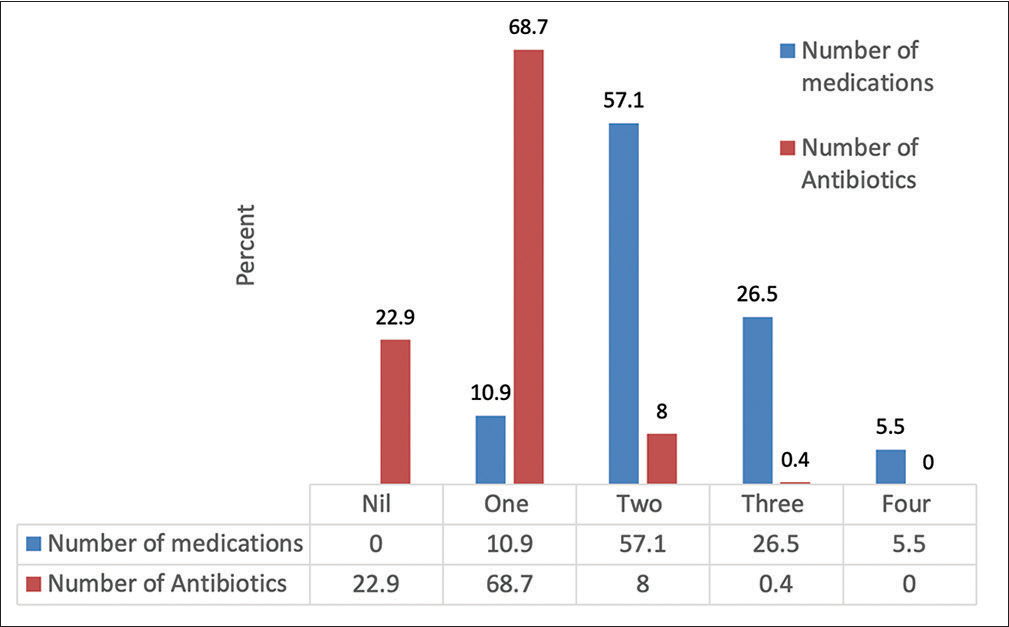
Medications were prescribed in all cases assessed. Thirty (7.9%) prescriptions contained one medication, 157 (57.1%) contained two medications, 73 (26.5%) contained three medications, and 15 (5.5%) contained four medications. The average number of medicines prescribed was 2.27. Most (212; 77.1%) of the prescriptions contained antibiotic medication. Of the 212 prescriptions that contained antibiotics, 189 (68.7%) contained one antibiotic, 22 (8.0%) contained two antibiotics, and one (0.4%) contained three antibiotics. Sixty-three (22.9%) prescriptions had no antibiotic medication [Figure 1].

- Number of medications and antibiotics prescribed.
Antihistamines (110; 40.0%) were the second most prescribed medications, followed by vitamins (75; 27.3%), antimalarials (55; 20%), minerals (38; 13.8), NSAIDS (35; 13.5), and analgesics (35; 12.7%). Other medications prescribed include cough syrup, anthelmintics, and bronchodilators [Figure 2].
- Medications prescribed for upper respiratory tract infection.
Specific antibiotic prescriptions
Seven classes of antibiotics were prescribed: Cephalosporins, macrolides, quinolones, fluoroquinolones, nitroimidazoles, trimethoprim/sulfamethoxazole, and penicillin. Table 3 shows the names of antibiotics as prescribed, the frequency of prescription, and combinations. There were 22 different prescription types found among the 275 cases assessed. The most common antibiotic prescribed was amoxicillin/clavulanate (79; 28.7%) followed by azithromycin (38; 13.8%).
| Antibiotic | Frequency (%) n=212 |
|---|---|
| Augmentin | 79 (37.2) |
| Azithromycin | 38 (17.9) |
| Cefuroxime (Zinnat) | 27 (12.7) |
| Cefixime | 14 (6.6) |
| Amoxil | 13 (6.1) |
| Cefpodoxime (Orelox) | 6 (2.8) |
| Septrin | 4 (1.9) |
| Ciprofloxacin | 3 (1.4) |
| Erythromycin | 3 (1.4) |
| Ofloxacin | 1 (0.5) |
| Cefuroxime, Erythromycin | 4 (1.9) |
| Augmentin, Azithromycin | 4 (1.9) |
| Cefuroxime, Azithromycin | 3 (1.4) |
| Augmentin, Cefpodoxime | 1 (0.5) |
| Augmentin, Erythromycin | 1 (0.5) |
| Cefuroxime, Clarithromycin | 1 (0.5) |
| Cefpodoxime (Orelox), Flagyl | 2 (0.9) |
| Cefpodoxime, Azithromycin | 2 (0.9) |
| Azithromycin, Septrin | 1 (0.5) |
| Cefixime, Azithromycin | 2 (0.9) |
| Cefuroxime, Azithromycin, Septrin | 1 (0.5) |
There was no statistically significant association between age category of child and the number of medications prescribed, whether a patient was prescribed antibiotics, and number of antibiotics prescribed (P > 0.05).
DISCUSSION
This study was conducted to assess the pharmacotherapy of URTIs in children 5 years and below in a tertiary institution. The study clearly shows the decreasing occurrence of URTIs with age which agrees with other authors.[21]
Our study found that 53.1% of the study sample with URTI were children under 24 months which is lower than found in a similar study where 85.0% were under 24 months.[22] Our study found higher proportion of occurrence in the 0–12 months (33.1%) and 12–60 months (60.8%) than that found in Punjab, India where the proportion of children with URTI was 20.0% in 1 day–12 months and 51.0% in the 12–60 months age group.[23] Other authors have shown that there is higher risk of acute RTIs in younger children.[21,22] This assertion agrees with our results where the frequency of URTI presentation tapered with increasing age.
More than half (57.1%) of our study population were male children. This is like other URTIs studies conducted in Enugu, Nigeria; Punjab, India; and Lao, PDR, where 61.7%, 55.5%, and 53.9%, respectively, were male children.[23-25] While the reason for the presence of more males may not be explained in these studies, some authors have found that there are more male child visits to hospital outpatient departments in their study settings.[19,26] Other authors in southeastern Nigeria have concluded that the male child tends to get more attention than the female especially when it comes to health as the Igbo tribe in general and the women specifically have a strong perception of the male child.[27]
Non-specific URTI was the most diagnosed URTI followed by tonsillitis among the study sample. Seventeen different classes and types of medications were prescribed for URTIs in this study with an average of 2.27 per case and 68% having a maximum of two medications. The average number of medications per case falls short of <2 recommended by the WHO indicator for rational prescribing and less than the case of the study in India which had an average of 3.4 with more than 75% of their study sample having more than two medications per prescription.[24,28] This is an indication of polypharmacy, increased risks of drug-drug interactions, and increased health-care expenses.
The prescription of antihypertensives, benzyl benzoate, antimalarials, antifungals, and anthelmintics shows inappropriate prescribing as these have no indication in the management of URTIs without comorbidities. Other authors have encountered prescriptions that have no indication for the documented medical conditions, and the reasons for this pattern of prescribing remain unclear.[19] The clinical implications of inappropriate prescribing remain the issue of drug therapy, specifically the exposure of the child to unnecessary medications and the inadvertent experience of adverse effects.
Our findings showed that antibiotics were the most frequently prescribed medications (77.1%) for URTIs and are distantly followed by antihistamines, vitamins, and antimalarials. This is far removed from the WHO standard of <30% maximum prescription of antibiotics for any drug utilization study.[28]
This value is also higher than in similar studies conducted in China and India where antibiotic prescriptions for URTI were 27.1% and 12.0%, respectively, but lower than found in the study conducted in Ibadan, Southwest Nigeria where 98.9% of the study population received antibiotics prescription for URTIs.[23,29,30] The study conducted in China and India showed rational use of antibiotics since they had a lower value than recommended by the WHO. Antibiotics are not routinely indicated for URTIs since the majority are of viral origin, self-limiting and, therefore, resolve on their own without antibiotics. The use of antibiotics is only recommended if laboratory tests confirm bacterial-induced URTI and would therefore be beneficial to the patient. Inappropriate prescribing of antibiotics fuels antimicrobial resistance which is now a public health threat. Only 10 (3.6%) of the study sample undertook laboratory tests with only six tests directly connected to URTIs unlike the study conducted in Ibadan where laboratory tests confirmed the presence of bacterial infection in 42.2% of the cases that received an antibiotic prescription. The inappropriate and empiric use of antibiotics for other childhood illnesses including diarrhea has also been confirmed in other studies.[19,25,31]
Seven classes of antibiotics were prescribed in 22 different ways either alone or in combination. The three most prescribed antibiotics were amoxicillin/clavulanate (37.3%), azithromycin (17.9%), and cefuroxime (12.7%), and this finding is different from the study conducted in Punjab (azithromycin; 61% amoxicillin/clavulanate; 31%, and cefuroxime; 5%).[23] It is noteworthy that the use of antibiotics for URTIs is not standardized in existing guidelines. Many of the antibiotics prescribed in our study are suitable for specific bacterial URTIs, but it remains unclear how the decision to prescribe them was reached.
The assessment of pharmacotherapy in our study showed that the prescriptions did not follow the guidelines which indicate that certain antibiotics be used only after laboratory culture and sensitivity tests.[8] A systematic review conducted with 13 guidelines found huge differences concerning categorization of URTIs and recommendations.[32] The authors concluded that future guidelines should use a consistent grading system for the quality of evidence and strength of recommendations. Furthermore, other authors confirmed that the management of childhood URTIs involves complex interactions of emotional and psychological factors influencing the decision-making process of the primary care providers; hence, a team care approach between caregivers and the health providers is vital to facilitate better management of URTIs in children.
The prescription of antihistamines (40.0%) would have been for symptomatic management of the discomfort experienced by patients with URTIs. This is much higher than that found in the study conducted by Tiwari et al. in India where only 9.0% received antihistamines. There needs to be a workable guideline for the management of URTIs in children.
CONCLUSION
The study revealed that antibiotics were the mainstay for the management of URTIs in the study center. We consider the utilization of antibiotics for such infections unnecessary and not indicated for the management of URTIs in children as culture and sensitivity tests were not done before initiation of antibiotic therapy. Our findings show that there is a need for strict implementation of the guidelines and initiation of antibiotic stewardship programs to curb the excessive use of antibiotics.
Acknowledgment
We acknowledge and appreciate the administrative officers and matrons of the pediatric unit who supported our data collection process.
Declaration of patient consent
Patient’s consent is not required as patient’s identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
None.
References
- The global impact of respiratory disease (2nd ed). Sheffield: European Respiratory Society; 2017.
- [Google Scholar]
- Prevalence of acute respiratory infection among under-five children in urban and rural areas of Puducherry, India. J Nat Sci Biol Med. 2015;6:3-6. doi:10.4103/0976-9668.149069
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence and factors associated with acute respiratory infection among under-five children in selected tertiary hospitals of Kathmandu Valley. PLoS One. 2022;17:e0265933. doi:10.1371/journal.pone.0265933
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of the acute respiratory infections and associated factors in the rural areas and urban slum areas of Western Maharashtra, India: A community-based cross-sectional study. Front Public Health. 2021;9:723807. doi:10.3389/fpubh.2021.723807
- [CrossRef] [PubMed] [Google Scholar]
- Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet. 2015;385:430-440. doi:10.1016/S0140-6736(14)61698-6
- [CrossRef] [PubMed] [Google Scholar]
- Respiratory infections In: Keystone JS, Korzarky PE, Leder K, eds. Travel medicine (4th ed). Netherlands: Elsevier; 2019. p. :527-537.
- [CrossRef] [Google Scholar]
- Antibiotic therapy of respiratory tract infections in children aged 3 to 6-theory and everyday practice. Fam Med Prim Care Rev. 2014;16:333-335.
- [Google Scholar]
- Upper respiratory tract infection medication. https://emedcine.medscape.com/article/302460-medication [Last accessed on 2022 Apr 04]
- [Google Scholar]
- Burden of acute respiratory tract infections as seen in university of Port Harcourt teaching hospital Nigeria. J US China Med Sci. 2015;12:158-162. doi:10.17265/1548-6648/2015.04.003
- [CrossRef] [Google Scholar]
- Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873. doi:10.1093/jac/dkx500
- [CrossRef] [PubMed] [Google Scholar]
- Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother. 2018;73(Suppl 2):ii36-ii43. doi:10.1093/jac/dkx500
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic prescription among outpatients in a prefecture of Japan, 2012-2013: A retrospective claims database study. BMJ Open. 2019;9:e026251. doi:10.1136/bmjopen-2018-026251
- [CrossRef] [PubMed] [Google Scholar]
- Association between national treatment guidelines for upper respiratory tract infections and outpatient pediatric antibiotic use in France: An interrupted time-series analysis. J Pediatr. 2020;216:88-94.e4. doi:10.1016/j.jpeds.2019.09.017
- [CrossRef] [PubMed] [Google Scholar]
- Antibiotic prescribing for children with upper respiratory tract infection: A Finnish nationwide 7-year observational study. Eur J Pediatr. 2022;181:2981-2990. doi:10.1007/s00431-022-04512-w
- [CrossRef] [PubMed] [Google Scholar]
- The socioeconomic burden of antibiotic resistance in conflict-affected settings and refugee hosting countries: A systematic scoping review. Confl Health. 2021;15:21. doi:10.1186/s13031-021-00357-6
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS One. 2017;12:e0189621. doi:10.1371/journal.pone.0189621
- [CrossRef] [PubMed] [Google Scholar]
- The use of unprescribed antibiotics in management of upper respiratory tract infection in children in Enugu, South East Nigeria. J Trop Pediatr. 2014;60:249-252. doi:10.1093/tropej/fmt111
- [CrossRef] [PubMed] [Google Scholar]
- Prescribing practices in the management of childhood diarrhoea in primary health care centres in a sub-urban community in Nigeria. J Community Med Prim Health Care. 2019;31:31-39.
- [Google Scholar]
- Upper respiratory tract infection. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532961/2022 [Last accessed on 2022 Jun 11]
- [Google Scholar]
- Household environment and symptoms of childhood respiratory infections in Nigeria, 2003-2013: A decade of progress and stagnation. BMC Infect Dis. 2018;18:296. doi:10.1186/s12879-018-3207-5
- [CrossRef] [PubMed] [Google Scholar]
- Management of acute respiratory infections in children under5 by self-medication and prescription of antibiotics. Int J Trop Dis Health. 2020;40:1-10. doi:10.9734/ijtdh/2019/v40j430234
- [CrossRef] [Google Scholar]
- Prescription practice in patients of upper respiratory tract infection at a pediatric outpatient clinic in Punjab. Indian J Pharm Pract. 2014;7:27-32. doi:10.5530/ijopp.7.2.6
- [CrossRef] [Google Scholar]
- Antibiotic prescription for under-fives with common cold or upper respiratory tract infection in Savannakhet province, Lao PDR. Trop Med Health. 2019;47:16. doi:10.1186/s41182-019-0143-z
- [CrossRef] [PubMed] [Google Scholar]
- Empiric antibiotic prescription among febrile under-five children in the university of Port Harcourt teaching Hospital, rivers State, Nigeria. Niger J Paediatr. 2014;41:234-238. doi:10.4314/njp.v41i3.16
- [CrossRef] [Google Scholar]
- Prescription of medicines for the management of childhood acute watery diarrhoea at a tertiary hospital in Ebonyi State, Nigeria. Niger J Pharm. 2022;56:128-135. doi:10.51412/psnnjp.2022.14
- [CrossRef] [Google Scholar]
- Perception of male gender preference among pregnant igbo women. Ann Med Health Sci Res. 2014;4:173-178. doi:10.4103/2141-9248.129027
- [CrossRef] [PubMed] [Google Scholar]
- How to investigate drug use in health facilities. 1993. Available from: https://apps.who.int/medicinedocs/pdf/s2289e/s2289e.pdf [Last accessed on 2021 May 09]
- [Google Scholar]
- Antibiotic prescriptions for children younger than 5 years with acute upper respiratory infections in China: A retrospective nationwide claims database study. BMC Infect Dis. 2021;21:339. doi:10.1186/s12879-021-05997-w
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of antibiotic prescriptions and use in under-five children in Ibadan, SouthWestern Nigeria. Afr Health Sci. 2018;18:1189-1201. doi:10.4314/ahs.v18i4.40
- [CrossRef] [PubMed] [Google Scholar]
- Drug prescribing pattern for under-fives in a paediatric clinic in South-Western Nigeria. Ethiop J Health Sci. 2015;25:73-78. doi:10.4314/ejhs.v25i1.10
- [CrossRef] [PubMed] [Google Scholar]
- Systematic review of evidence-based guidelines on medication therapy for upper respiratory tract infection in children with AGREE instrument. PLoS One. 2014;9:e87711. doi:10.1371/journal.pone.0087711
- [CrossRef] [PubMed] [Google Scholar]







