Translate this page into:
Antipsychotic initiation in mechanically ventilated patients in a medical intensive care unit
*Corresponding author: Hannah R. Ritchie, Pharm.D. Department of Pharmacy Services, Baystate Medical Center, Springfield, Connecticut, United States. hannah.rae.ritchie@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Ritchie HR, Hodle TJ, Spinner HE. Antipsychotic initiation in mechanically ventilated patients in a medical intensive care unit. Am J Pharmacother Pharm Sci 2024:1.
Abstract
Objectives:
Guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients (PADIS) in the intensive care unit (ICU) promote use of analgosedation to minimize pain, reduce anxiety, and facilitate care. They also suggest against routine use of antipsychotics (APs) for delirium. Our institution’s adaptation incorporates assessment-driven, protocol-based pain, and sedation management and suggests a short course of APs in patients with agitated delirium, defined as Confusion Assessment Method for the ICU (CAM-ICU) positive with Richmond Agitation Sedation Scale (RASS) ≥ +2. While the use of APs in the ICU is typically for delirium, a recent study assessed whether quetiapine reduced sedative requirements among non-delirious patients. The purpose of this study was to assess adherence to our institutional guideline for AP use and to describe sedative and opioid use in relation to AP initiation.
Materials and Methods:
This retrospective study included patients who were mechanically ventilated and received ≥ 3 new start AP doses. The primary outcome was adherence to our guideline for use of APs in agitated delirium. The secondary outcomes were CAM-ICU and RASS scores in relation to AP initiation and change in sedative and analgesic infusion rates following AP initiation.
Results:
Thirty-eight patients were included in the study. Five had APs initiated appropriately per our guideline. There was no clinically significant change in continuous infusion rates in the 24 h before and after AP initiation.
Conclusion:
Overall, AP use was liberal with patients being started on APs who did not have agitated delirium, thus indicating potential alternative indications for initiation. APs did not result in a clinically significant change in continuous infusion requirements in the 24 h following initiation.
Keywords
Antipsychotics
Delirium
Sedation
Pain
Critically ill
INTRODUCTION
Mechanically ventilated patients in the intensive care unit (ICU) often require sedative medications to reduce anxiety, decrease stress, and facilitate care. However, many studies have demonstrated the adverse effects of deep levels of sedation in ICUs, such as longer ventilator time, prolonged ICU length of stay (LOS), increased risk of delirium, slower cognitive and physical recovery, and higher rates of mortality.[1-3] Society of Critical Care Medicine’s Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in adult patients in the ICU promote the use of light sedation, rather than deep sedation, to improve patient outcomes.[2] Further, the PADIS guideline recommends against routine use of antipsychotics (APs) to prevent or treat delirium. Delirium is a frequently encountered diagnosis occurring in up to 80% of critically ill adult patients.[4,5] Delirium, like deep sedation, may result in longer duration of mechanical ventilation, increased ICU LOS, slower cognitive recovery, and increased mortality.[4,6] Delirium in the ICU also increases the likelihood of being discharged to a post-acute care facility, may increase cost of care, and is a major risk factor for the development of post-intensive care syndrome.[7,8] The use of APs in the management of ICU delirium is a known practice, and the risks of ICU delirium must be balanced with the risk of the use of APs. Our institution’s adaptation of the PADIS guideline recommends a short course of AP therapy in patients who are delirious and agitated, defined as Confusion Assessment Method for the ICU (CAM-ICU) positive with Richmond Agitation Sedation Scale (RASS) score ≥ +2 [Figure 1].[9] The adapted recommendations also suggest short courses of continuous infusion sedatives as management for agitation and inadequate sedation. The use of APs as adjunct sedation has emerged as an area of interest that has only minimally been discussed. A recent study assessed whether adjunctive use of quetiapine reduced sedative dosage requirements among 57 mechanically ventilated medical ICU (MICU) adults without delirium.[10] Ultimately, Ohman et al. concluded that the use of quetiapine at a median dose of 25 mg every 12 h did not result in a significant reduction in sedative requirements 24- or 48-h following initiation.[10] In addition to the recent interest in the effects of APs on sedation, the coronavirus disease (COVID-19) pandemic has changed sedation management and AP use in the ICU. Recommendations were provided early in the pandemic to discontinue therapeutically potent sedatives as soon as possible or replace them with agents that do not suppress the respiratory drive, such as APs or alpha-2 agonists.[11] Although these recommendations were likely affected by medication shortages and other constraints caused by the pandemic, it is possible they influenced general ICU practice or that providers were using similar approaches in patients without COVID-19 despite the lack of evidence to support transitioning from potent sedatives to alternative agents.[12] Overall, there are many factors, as described, contributing to decision-making on the use of APs in critically ill patients and the purpose of our analysis was to further explore this area of drug utilization in our ICU. The aim of this study was to describe the use of AP therapy in critically ill, mechanically ventilated adults in the MICU of a tertiary academic medical center by assessing adherence to an institution-specific guideline for delirium management and the effects of APs on continuous infusion sedative and analgesic agents.
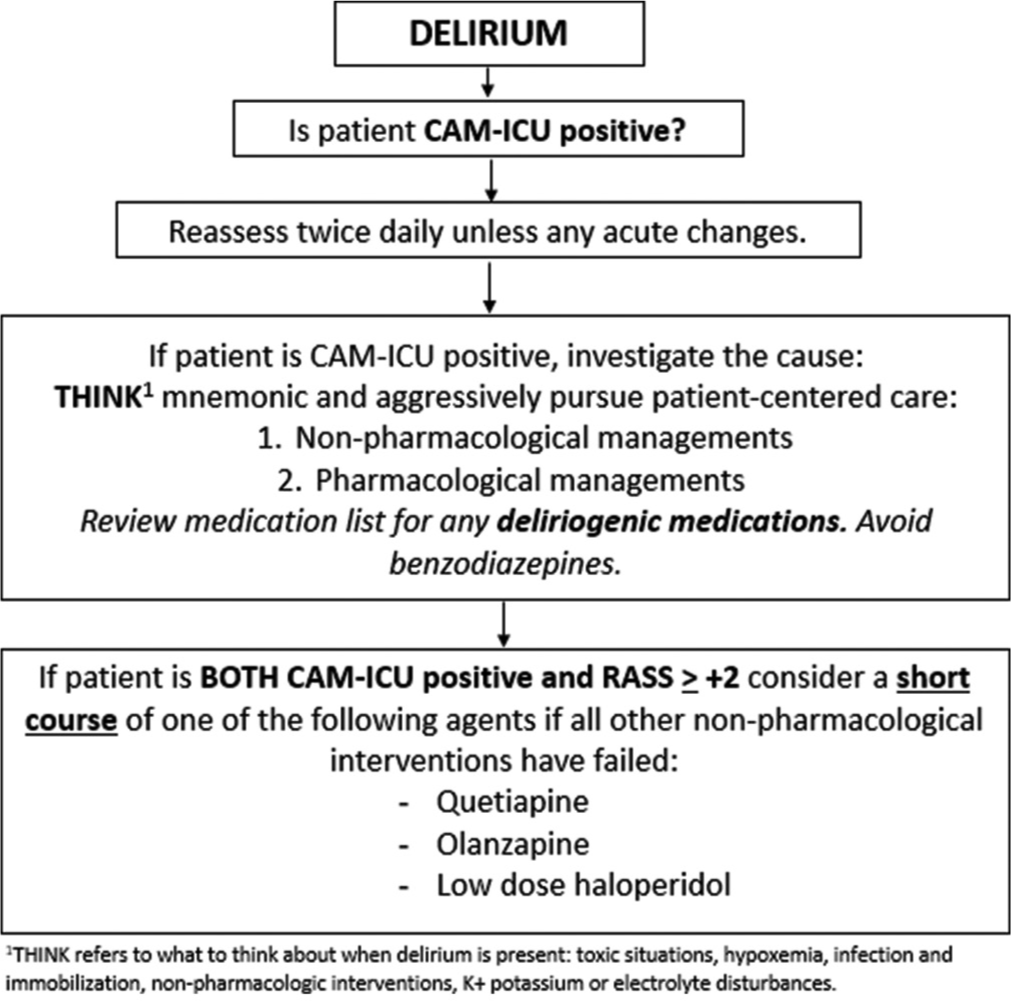
- Baystate Medical Center’s PADIS adaptation.
MATERIALS AND METHODS
Design and setting
This retrospective comparator study was conducted at Baystate Medical Center (BMC), a 716-bed independent academic medical center and Level 1 Trauma Center located in western Massachusetts. BMC has six ICUs including a MICU. This study included patients 18 years or older admitted to the MICU who had quetiapine, olanzapine, or haloperidol ordered between June 2020 and June 2021, received at least 3 doses of APs, and were mechanically ventilated at time of initiation. Patients were excluded if they met any of the following criteria: underlying psychiatric disorder necessitating outpatient AP use, initiation of APs on another inpatient unit before or following MICU admission, active neuromuscular blockade (NMB) within 24 h of initiation, intentional extubation <24 h after initiation, underwent an operating room procedure within the 24 h before or after initiation, or active COVID-19 infection. APs were initiated at provider discretion and were retrospectively assessed for adherence to our institution-specific guideline [Figure 1]. Continuous infusion sedatives and opioids were also ordered per provider discretion. Critical Care Pain Observation Tool (CPOT) goals were designated by providers for continuous infusion sedatives and opioids, respectively, and infusions were titrated to these goals per nursing-driven protocols. This goal-directed approach included spontaneous awakening trials as appropriate. RASS scores were charted hourly, while CPOT and CAM-ICU assessments were completed once per every 12-h nursing shift or more frequently if patient condition changed. This study was determined to be non-human subjects research by the BMC institutional review board.
Measures
The objectives of this study were, first, to describe the use of APs in mechanically ventilated adults in the MICU in relation to guideline and institutional recommendations and, second, to characterize the effects of APs on continuous infusion sedation and analgesia. The primary outcome was adherence to an institutional guideline for the use of APs in critically ill patients with agitated delirium. Appropriate use was defined per the guideline [Figure 1] as AP initiation in patients who were CAM-ICU positive with RASS ≥ +2. Secondary outcomes included CAM-ICU and RASS scores at 24 h before, time of, and 24 h after AP initiation, change in sedative and analgesic infusion rates from the 24 h before initiation to the 24 h following initiation, total daily dose of opioids before and after initiation, rate of unplanned extubations, change in ventilator settings, rate of AP continuation at ICU and hospital discharge, and incidence of QTc prolongation following AP initiation. RASS, CAM-ICU, and CPOT scores were recorded at 24 h before, time of, and 24 h following AP initiation with a ± 8-h window for each given that CAM-ICU and CPOT scores were typically recorded once per 12-h shift. Ventilator settings were recorded at time of AP initiation and for up to 12 h following initiation. QTc prolongation was assessed using the most recent electrocardiogram (ECG) before and following initiation, if available, with no specified time frame.
Data source and data collection
Patients 18 years or older admitted to the MICU who had quetiapine, olanzapine, or haloperidol ordered between June 2020 and June 2021 were identified through TheraDoc, an electronic clinical surveillance application. Patients were then screened through retrospective chart review of the electronic medical record for additional inclusion and exclusion criteria. Data were collected for included patients through retrospective chart review and were entered into a research electronic data capture database. All data were collected and calculated manually, except for total daily dose of opioids which were collected using Elimu Informatics morphine milliequivalent (MME) monitoring application within the Cerner electronic system.
Statistical analysis
Data are reported as medians with interquartile range (IQR) or means with standard deviation (SD). For medication data, both weight-normalized dosages (mg/kg or mcg/kg) and total dosages (mg or mcg) were recorded. Statistical analyses were not performed given the small sample size and the expectation that results were more likely to be clinically significant than statistically significant.
RESULTS
Patient demographics
From June 2020 to June 2021, a total of 388 patients had an AP order associated with the MICU location. Of these 388 patients, 38 (9.8%) met eligibility criteria and were included in the study cohort [Figure 2]. Baseline characteristics are shown in Table 1. Patients had a median age of 64 years and average weight of 79.9 kg. Females constituted 57.9% of the population and reasons for admission included respiratory illness (50.0%), infection (34.2%), and neurologic illness (28.9%). Thirty-seven patients (97.4%) received quetiapine and one patient (2.6%) received olanzapine as their primary APs, respectively. The median (IQR) dose of quetiapine patients received in the 24 h following initiation was 50 (37.5–75) mg. The patient who received olanzapine was administered 10 mg in the 24 h following initiation.
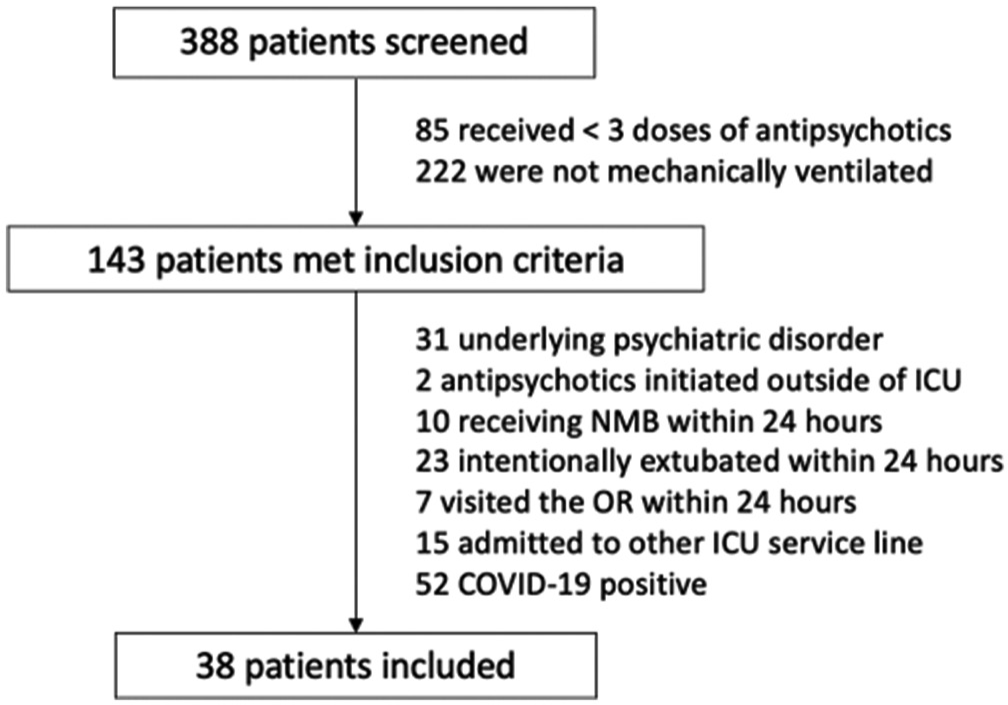
- Patient selection CONSORT diagram.
| Baseline characteristic | n=38 |
|---|---|
| Age (years) | 64 (53.5–73.8) |
| Gender (female) | 22 (57.9) |
| Weight (kg) | 79.9 (60.9–92.2) |
| Admission diagnosis/reason for admission | |
| Neurologic illness | 11 (28.9) |
| Respiratory illness | 19 (50) |
| Cardiac illness | 4 (10.5) |
| Digestive or urologic illness | 1 (2.6) |
| Hepatic disease | 1 (2.6) |
| Renal disease | 1 (2.6) |
| Infection | 13 (34.2) |
| Hematologic/oncologic illness | 2 (5.3) |
| Hemodynamic compromise | 3 (7.9) |
| Other | 1 (2.6) |
| On vasopressors at time of AP2initiation | 8 (21.1) |
| QTc (ms, Bazett) | 446 (424–475) |
Guideline adherence
Of the 38 patients who received APs, only five (13.2%) patients were CAM-ICU positive with RASS ≥ +2 at time of AP initiation. CAM-ICU and RASS scores at 24 ± 8 h before initiation, time of initiation (± 8 h), and 24 ± 8 h following initiation are shown in Figure 3. Patients were primarily CAM-ICU positive at all three assessment times. RASS scores ranged from −4 to +3, with patients primarily RASS +1 at 24 h prior, RASS −2 at time of initiation, and RASS −1 at 24 h after AP initiation.
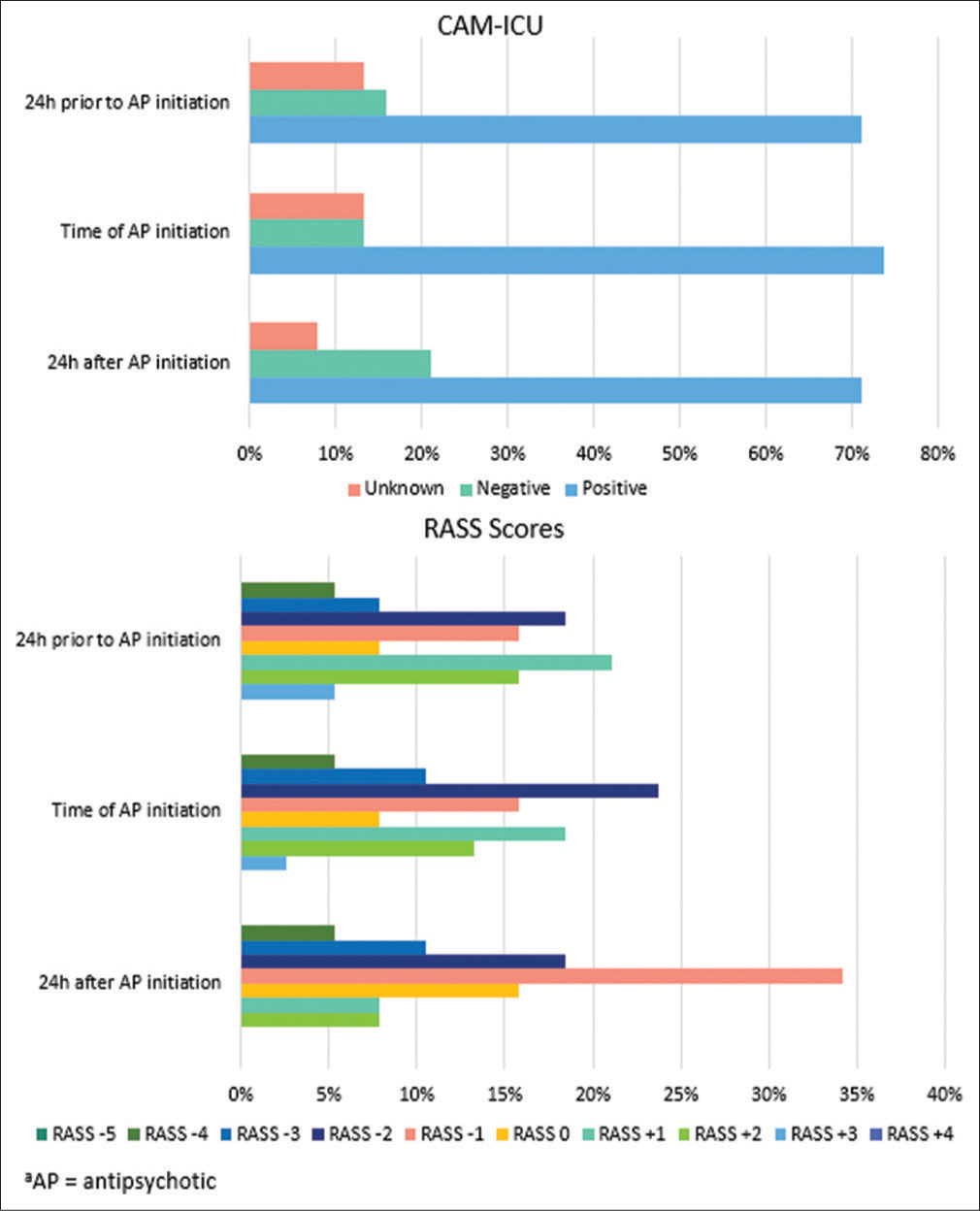
- Confusion assessment method for the intensive care unit and Richmond Agitation Sedation Scale scores.
Sedative and opioid utilization
The most common sedative agents used in the study cohort were propofol and dexmedetomidine with 23 and 21 patients receiving each agent, respectively. There were three patients each on midazolam and ketamine infusions. The most common continuous analgesic agent was fentanyl, with 19 patients receiving continuous infusions. Only three patients received continuous infusion hydromorphone. Changes in continuous infusion sedative and analgesic rates are shown in Figure 4. In patients who received propofol, the average (±SD) rate before AP initiation was 16.8 ± 15.8 mcg/ kg/min while the average rate following initiation was 16.7 ± 14.5 mcg/kg/min. In patients who received dexmedetomidine, the average (±SD) rates in the 24 h before and following AP initiation were the same at 0.7 ± 0.5 mcg/kg/h. For midazolam, patients received an average (±SD) of 1.3 ± 0.6 mg/h in the 24 h before initiation compared to 0.4 ± 0.2 mg/h in the 24 h following. Finally, in the patients who received ketamine, the average (±SD) rates before and after were 0.9 ± 0.6 mg/kg/h and 0.5 ± 0.5 mg/kg/h, respectively. Rates (±SD) of fentanyl before and after initiation were 81.1 ± 40.6 mcg/h and 76.0 ± 52.0 mcg/h and rates (±SD) of hydromorphone were 4.4 ± 3.4 mg/h and 3.8 ± 2.7 mg/h, respectively. Overall, there was no clinically significant difference in rates of continuous infusion sedatives or analgesics in the 24 h before and after AP initiation. Despite minimal changes in continuous infusion analgesics, patients’ CPOT scores decreased over the study period. Most patients (63.1%) had CPOT scores <2 at 24 h before AP initiation. At time of initiation, 73.7% were documented as CPOT <2 and at 24 h following initiation, 86.9% had CPOT scores <2. Total daily dose of opioids, collected and reported as MMEs, decreased by an average of 2.5% in the overall cohort. Further description of patients’ RASS and CAM scores is provided in Figure 3.
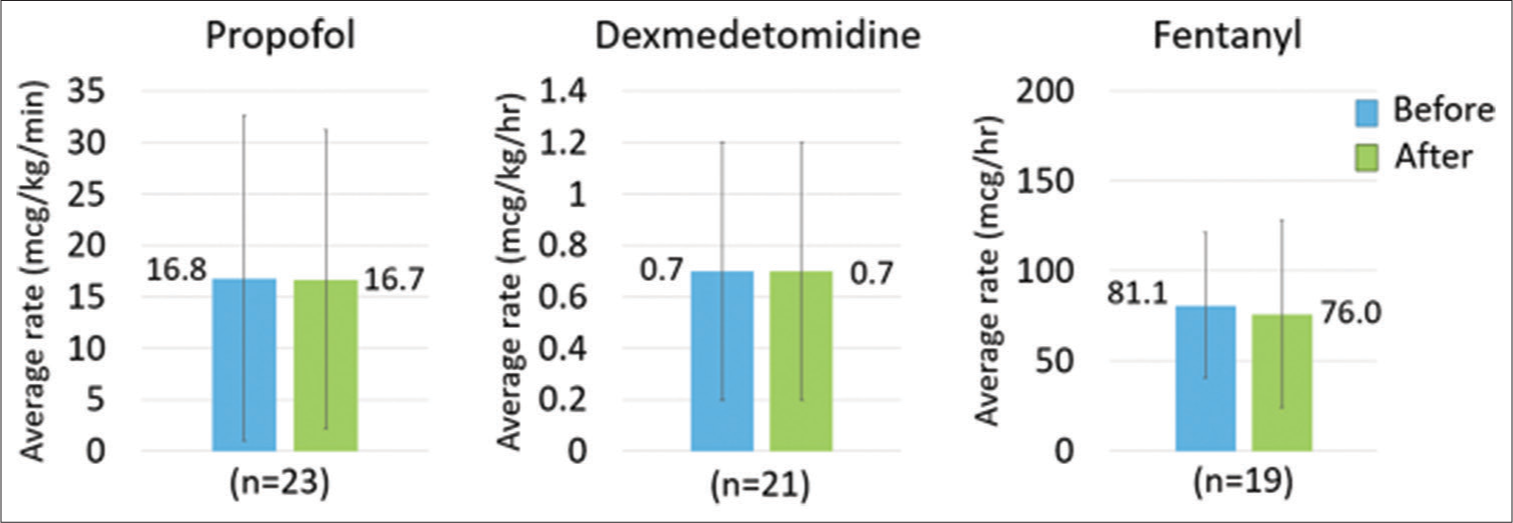
- Change in continuous infusion sedative and analgesic medications.
Additional secondary outcomes
The median (IQR) AP days of therapy was 7.7 (3.8–11.1) days. There were zero patients who had an unplanned extubation documented in the 24 h following AP initiation. Eight (21.1%) patients had a change in ventilator settings following AP initiation, defined loosely as a transition from assist control ventilation to pressure support ventilation (PSV) and sustained on PSV for 12 or more hours. The median (IQR) time on the ventilator was 9.9 (5.7–20.9) days. The median (IQR) ICU and hospital LOS were 11.3 (6.9–22.9) and 20.8 (12.8–34.5) days, respectively. Eleven (29.7%) patients experienced ICU mortality and an additional 5 (13.5%) patients expired in the hospital after ICU discharge.
Safety
Patients had a median (IQR) baseline QTc value of 446 (424– 475) [Table 1] and 18.4% experienced prolonged QTc, defined as QTc >500 ms on repeat ECG, following AP initiation. Thirteen (34.2%) and 4 (10.5%) patients had APs continued at time of discharge from the ICU and hospital, respectively.
DISCUSSION
In this study, we found adherence to institution-specific recommendations for the use of APs in mechanically ventilated adults with hyperactive delirium to be low with many patients being initiated on APs who were not CAMICU positive with RASS ≥ ±2. Most patients who received APs were CAM-ICU positive with RASS -2 to +1 at time of initiation [Figure 3]. In addition to our main finding, we also found that APs did not appear to have a clinically significant effect on rates of continuous infusion sedatives or analgesics in the 24 h following initiation. Changes in depth of sedation and level of pain also did not appear to be largely different, though fewer patients seemed to be agitated based on RASS scores 24 h after the first dose of APs. Finally, we found that many patients were continued on APs at time of ICU and hospital discharge. A prior review conducted at BMC indicated that 16.3% of patients were continued on APs at time of hospital discharge. While the present study indicates progress in AP discontinuation with only 10.5% of patients discharged from the hospital on new start APs, there is still room for improvement. Our findings indicate that APs were being used in patients without agitated delirium, suggesting potential alternate prescribing indications such as adjunct sedation. The initiation of APs should be done with some precautions, and further description of the potential benefits and risks as described in this study will help providers in discerning appropriate use of APs.
The impact of APs on sedative and analgesic requirements has only been explicitly evaluated in one study.[10] In the retrospective intrapatient comparator analysis by Ohman et al., the authors found that the use of quetiapine as an adjunct sedative was not associated with reduced dosage requirements of dexmedetomidine, propofol, or midazolam at 24- and 48-h following AP initiation.[10] There was also no statistically significant difference in the absolute dose of opioids.[10] While the use of quetiapine as adjunct sedation and analgesia has only been discussed in one study, to our knowledge, the effects have been described in prior studies evaluating the use of APs for delirium and delirium prophylaxis. Results from these prior studies have been mixed, with smaller prospective studies describing fewer sedative and opioid days and larger, randomized trials failing to find significant effects of APs on sedation.[13-16] There is significant heterogeneity among these studies and despite some studies having similar AP dosing and titration protocols, there is a lack of agreeance regarding the effects of various APs on benzodiazepine, opioid, propofol, and dexmedetomidine exposure.[13-16] Assessment of the effects of APs on analgesia is confounded by the fact that CPOT assessment involves many components that are affected by delirium. Patients may have a CPOT score of two or higher from actions such as fighting the ventilator or body movements including attempting to sit up, pulling at tubes, and thrashing limbs.[17] There is significant overlap between the presentations of delirium and pain in mechanically ventilated, sedated patients. Given the conflicting evidence and clinician desire to reduce the amounts of sedatives and opioids patients receive, it is possible that APs were being initiated in this cohort as adjunct sedation. We hypothesize that the indication for APs was often for adjunct sedative therapy as demonstrated by the low rates of initiation for agitated delirium described in our study. The prevalence of this clinical practice is relevant, and the use of APs as adjunct sedation should be further investigated at our institution and beyond.
While it is difficult to quantify the effects of the COVID-19 pandemic on ICU management of non-COVID patients, we suspect that the adjusted management of PADIS in patients with COVID-19 has affected our management of patients without the viral infection. The question of what effects the pandemic has had on critically ill patients without COVID-19 has yet to be elucidated but is an area that would help further explain the findings of our study. The increased use of APs has been described in other populations since the pandemic began, raising the possibility that COVID-19 has led to increased prescribing rates of APs in the critically ill population, too.[18,19] We suspect that AP utilization as adjunct sedation has increased in attempts to wean patients with acute respiratory distress syndrome from COVID-19 from the high amounts of sedatives and opioids they require; we question if this practice has potentially affected the nonCOVID critically ill population.
Although we find the results from our study to be clinically relevant, we acknowledge that there are many limitations. This is a single-center descriptive study with a small sample size that was not powered to show statistical significance and, therefore, external validity may be limited. All data were collected retrospectively which may have led to incomplete or inaccurate information. Only 10% of patients screened met inclusion criteria; extensive exclusion criteria were applied to try and account for potential confounding factors that would have affected patients’ level of sedation, odds of developing ICU delirium, and opioid requirements. We acknowledge that it is impossible to account for all potential confounding variables. Finally, patient condition changes frequently in the ICU and we recognize that retrospective data fails to account for all measures taken into consideration when managing patients’ sedation, agitation, and delirium. Patients who may have been agitated and delirious at time of AP ordering could have received interventions not accounted for in our data collection, thus making them appear to have lower RASS scores at time of AP administration. Although some patients may have been appropriate for APs at time of physician ordering and pharmacist verification, this may not have been captured in our data collection. Furthermore, while a goal-directed, nursing-driven approach to sedation was utilized in our MICU during the study period, adherence to sedation goals was assumed and compliance was not assessed. Sedation management was not standardized during the study period and frequent sedative titration, tapering, and transitions occurred, which is reflective of current clinical practice in the management of critically ill adults but could not be accounted for in this study. Finally, the frequency and timing of ECG assessment were not standardized nor were patients assessed for concomitant QT prolonging agents, thus affecting the accuracy and description of QTc prolongation in our study population. Strengths of this study include its pragmatic design, the exclusion of patients with important confounders, and assessment of both disease-oriented and patient-oriented outcomes. This novel study also shed light on the topic of APs as adjunct sedatives, which has only previously been discussed in one publication. The findings of this study have been shared with practitioners within our MICU, but we feel that this information should also be shared broadly to encourage others to assess indications for AP use in their critically ill patient populations.
CONCLUSION
Overall, these findings are an important contribution to practice at our institution as it relates to AP use and to the limited literature available describing the off-label use of APs as adjunct sedatives. The use of APs did not result in a clinically significant change in continuous infusion sedative and opioid requirements in the 24 h following initiation in mechanically ventilated adults with or without delirium. Clinicians in the ICU should reassess the indication and utility of APs in their patients frequently and should consider discontinuation if the AP is being used for an indication outside of ICU delirium. Future studies that examine the effects of APs on sedation prospectively using a standardized sedation approach would be useful as would studies assessing the changes in PADIS management of patients before and after the COVID-19 pandemic.
Ethical approval
The research is approved by the Baystate Medical Center Institutional Review Board, number BH-22-034, dated 01/20/2022.
Declaration of patient consent
Patient’s consent was not required as patients identity is not disclosed or compromised.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. There are no other contributors of significance to report.
References
- Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Crit Care. 2015;19:197. doi:10.1186/s13054-015-0929-2
- [CrossRef] [PubMed] [Google Scholar]
- Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.3410/f.733825200.793559140
- [CrossRef] [Google Scholar]
- Differential effects of gamma-aminobutyric acidergic sedatives on risk of post-extubation delirium in the ICU: A retrospective cohort study from a New England health care network. Crit Care Med. 2022;50:e434-e444. doi:10.1097/ccm.0000000000005425
- [CrossRef] [PubMed] [Google Scholar]
- Association of delirium with long-term cognitive decline: A meta-analysis. JAMA Neurol. 2020;77:1373-1381. doi:10.1001/jamaneurol.2020.2273
- [CrossRef] [PubMed] [Google Scholar]
- Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703-2710. doi:10.1001/jama.286.21.2703
- [CrossRef] [PubMed] [Google Scholar]
- Long-term outcomes in ICU patients with delirium: A population-based cohort study. Am J Respir Crit Care Med. 2021;204:412-420. doi:10.1164/rccm.202002-0320oc
- [CrossRef] [PubMed] [Google Scholar]
- The cost of ICU delirium and coma in the intensive care unit patient. Med Care. 2018;56:890-897. doi:10.1097/mlr.0000000000000975
- [CrossRef] [PubMed] [Google Scholar]
- Risk factors for post-intensive care syndrome: A systematic review and meta-analysis. Aust Crit Care. 2020;33:287-294. doi:10.1016/j.aucc.2019.10.004
- [CrossRef] [PubMed] [Google Scholar]
- Implementing delirium screening in the ICU: Secrets to success. Crit Care Med. 2013;41:2196-2208. doi:10.1097/CCM.0b013e31829a6f1e
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of quetiapine as a sedative adjunct in mechanically ventilated adults without delirium. Ann Pharmacother. 2021;55:149-156. doi:10.1177/1060028020944409
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020;24:176. doi:10.1177/1060028020944409
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19: ICU delirium management during SARS-CoV-2 pandemic-pharmacological considerations. Crit Care. 2020;24:375. doi:10.1186/s13054-020-03072-5
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of quetiapine in critically ill patients with delirium: A prospective, multicenter, randomized, double-blind, placebo controlled pilot study. Crit Care Med. 2010;38:419-427. doi:10.1097/ccm.0b013e3181b9e302
- [CrossRef] [PubMed] [Google Scholar]
- Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
- [CrossRef] [PubMed] [Google Scholar]
- Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: The MIND randomized, placebo-controlled trial. Crit Care Med. 2010;38:428-437. doi:10.1097/ccm.0b013e3181c58715
- [CrossRef] [PubMed] [Google Scholar]
- Quetiapine for delirium prophylaxis in high-risk critically ill patients. Surgeon. 2021;19:65-71. doi:10.1016/j.surge.2020.02.002
- [CrossRef] [PubMed] [Google Scholar]
- Impact of implementing the critical care pain observation tool on nurses' performance in assessing and managing pain in the critically ill patients. Indian J Crit Care Med. 2019;23:165-169. doi:10.5005/jp-journals-10071-23146
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of medication prescribing before and after the COVID-19 pandemic among nursing home residents in Ontario, Canada. JAMA Netw Open. 2021;4:e2118441. doi:10.1001/jamanetworkopen.2021.18441
- [CrossRef] [PubMed] [Google Scholar]
- Antipsychotic medication prescribing in long-term care facilities increased in the early months of the COVID-19 pandemic (issue brief) United States: Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services; 2022.
- [Google Scholar]







