Translate this page into:
Prospects of isolating new antimicrobial compounds from plants: The case of Azadirachta indica bark extract
*Corresponding author: Oluchi Judith Osuala, Department of Pharmaceutical Microbiology and Biotechnology, Madonna University, Elele, Rivers State, Nigeria. osualaoluchioo@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Osuala OJ, Igwe SE, Ezemba CC, et al. Prospects of isolating new antimicrobial compounds from plants: The case of Azadirachta indica bark extract. Am J Pharmacother Pharm Sci 2024:4.
Abstract
Objectives:
Essential oils and extracts from medicinal plants have been shown to have antimicrobial properties in several investigations carried out in regions with diverse floras. This study intends to evaluate the antimicrobial activity of Azadirachta indica (Neem plant) bark extract on microbial isolates.
Materials and Methods:
The plant’s bark was cut out of the tree, dried, and pulverized using a mechanical grinder. The crushed barks were split in half, one half macerated in ethanol and the other put through the Soxhlet apparatus. The ethanol extract of plant bark was used to analyze microbial isolates (Pseudomonas aeruginosa, Bacillus subtilis, Escherichia coli, Staphylococcus aureus, and Candida albicans). The active components in the extracts were analyzed using gas chromatography-mass spectrometry.
Results:
According to the inhibition zone width, mean inhibition concentration, and lowest bactericidal concentration, all organisms were shown to be sensitive to the antibacterial activities of A. indica at varied doses of the extracts utilized. For every isolate examined, the minimum inhibitory concentration (MIC) of the extract was 12.5 mg/mL; however, B. subtilis had a concentration of 25 mg/mL. The extract had bactericidal activity on all the isolates except Bacillus sp. The minimum bactericidal concentration (MBC) for the isolates was 12.5 mg/mL for P. aeruginosa, S. aureus, and C. albicans, and 100 mg/mL for E. coli. Among the principal compounds discovered are pentadecanoic acid, 14-methyl-methyl ester, stigmasterol, 9-octadecanoic acid (z)-methyl ester, methyl stearate, n-hexadecanoic acid, linoelaidic acid, and Vitamin E.
Conclusion:
Our research showed that the ethanol extract from A. indica bark contains several bioactive compounds with antimicrobial properties.
Keywords
Antimicrobial susceptibility testing
Azadirachta indica
Neem plant
antimicrobial agents
Bioactive compounds
INTRODUCTION
Bacteria are the primary causative agent in most infectious diseases.[1] Identification of microbial susceptibilities to various antibiotics has become essential with the availability of laboratory culture techniques for cultivating bacteria. This allows medical professionals to promptly start patients on the right therapeutic regimens.[2]
To combat microbial resistance, there has been a rise in interest in recent years in the discovery and synthesis of new antimicrobial chemicals sourced from various sources. Antimicrobial activity screening techniques have thus drawn more interest.
Plants might be a possible answer to the shortage of novel antibacterial agents and the fight against antibiotic resistance.[3,4] Numerous complex and structurally diverse compounds may be found in plants and other natural sources. As potential antibacterial agents, many research work have been focused on examining plant and microbial extracts, essential oils, pure secondary metabolites, and freshly synthesized chemicals.[5-7]
Historically, medicinal plants are utilized in many regions of the globe, where access to modern healthcare is restricted[8] and it has been posited that traditional medicines are used by about 80% of the world’s population.[9]
Azadirachta indica, commonly known as the neem plant, is mostly grown on the Indian subcontinent. Before the emergence of written history, humans utilized neem widely to cure a variety of diseases due to the numerous beneficial effects of their many constituents.
Annual antimicrobial resistance globally has continued to rise to about 750,000,[10] with a predicted increase to 10 million by 2050.[11] In tropical countries, infectious illnesses account for almost half of all fatalities. Even with breakthroughs in microbiology understanding and management, drug-resistant microbial outbreaks and the emergence of previously recognized disease-causing bacteria continue to pose serious public health risks in affluent nations.[12] This study aims to determine if the A. indica bark has an antimicrobial effect on the microbial isolates and also identify some of the organic compounds present in the extracts.
MATERIALS AND METHODS
Plant identification and collection
A. indica plant material was taken from the Department of Pharmacognosy Medicinal Garden at Madonna University Elele Campus in Rivers State, Nigeria. The plant material was authenticated at the medical plants herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Obafemi Awolowo University, Ile-Ife, and assigned a Voucher number: FPI 2475.
Extraction of plant’s active ingredients
The extraction of the plants was done as previously reported.[13,14] A. indica fresh bark was washed with purified water and air-dried for 14 days. A mechanical blender was used to pulverize the bark, and the particle size was further decreased using an electric grinding mill. 200 g of the powdered material was placed into a Winchester container containing 900 mL ethanol. The mouth of the bottle was closed with a lid to prevent solvent evaporation and then the powder was allowed to macerate for 72 h and then carefully filtered to separate the marc from the extract using a filter paper (Whatman Cat No 1001 150). The filtrate was then filtered again using filter paper transferred to a beaker and later concentrated in a 45°C-water bath for 20 min. For additional analysis, the extract was weighed and computed. The ethanol extract weighed 22.5 g giving a percentage yield of 11.25%.
To get the requisite fixed oils, the Soxhlet extraction method[13] was applied. 200 g of the powdered material was utilized. The solvent utilized was N-hexane (400 mL), a non-polar solvent with a boiling point of 68°C. The extraction procedure involves adding the grounded neem bar (200 g) into the extractor which is connected to a round bottom flask attached to the heat source. Four hundred milliliters of the solvent (n-Hexane) were poured into the connected round bottom flask, and the heat source was switched on and regulated to 68°C for evaporation of the solvent. The solvent on heating evaporates, passing through the condenser condenses into the extractor carrying the plant extract, and solubilizes the desired extract. The solvent continues to condense into the extractor until it reaches above the siphon bend on the extractor and the extract in a mixture with the solvent flows back to the connected round bottom flask through the siphon tube. The cycle continues for 48 h. After the extraction process, the mixture of the solvent and extract was allowed to evaporate without being heated so that the solvent can evaporate leaving the extract which was used for further analysis. The n-Hexane extract weighed 8.7 g giving a percentage yield of 4.35%.
Preparing the serial dilution
The ethanol extract was serially diluted using the twofold dilution procedure. Two grams of the ethanol extracts were weighed into beakers and diluted in 10 mL of dimethylsulphoxide (DMSO) to make a stock solution with a concentration of 200 mg/mL. Dilute solutions were prepared from the stock, and six test tubes were labeled A-F as follows A = 100 mg/mL, B = 50 mg/mL, C = 25 mg/mL, D = 12.5 mg/mL, E = 6.25 mg/mL, and F = 200 mg/mL. Ciprofloxacin (5 µg) and fluconazole (10 µg) were used as positive controls for bacteria and fungi isolates, respectively. The negative controls used were DMSO.
Test isolate confirmation
The isolates (Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, Escherichia coli, and Candida albicans) were subcultured from conserved agar slants onto selective media (media for bacteria) and Sabouraud Dextrose agar (all-purpose medium for fungus) for 24 h and 72 h, respectively, before use. Biochemical assays such as catalase test, coagulase test, oxidase test, indole test, lactophenol cotton blue staining, and Gram staining were also employed to confirm the laboratory isolates.[15] The duly identified pure cultures were standardized to an inoculum size of 1.5 × 108 CFU/mL which corresponds to the 0.5 McFarland standards. This was accomplished by diluting the cells to 0.08–0.1 optical density at 600 nm using a spectrophotometer.
Antimicrobial studies
The sensitivities of bacterial isolates to the extracts were tested using the agar well diffusion method – the punch-hole agar diffusion technique. To avoid contamination, Mueller– Hinton agar plates were prepared, and the isolates were meticulously swabbed with their designated swab sticks on the surface of the sterile plates. A sterile cork borer with an 8 mm diameter was used to drill holes in the solidified agar plates, and 50 mL of the extract was put into the bored holes.
Mueller–Hinton supplementary agar was prepared using Mueller–Hinton agar and supplemented with 2% glucose and 0.5 g/mL methylene blue to stimulate fungal growth in order to measure C. albicans susceptibility. The isolate of fungus was swabbed over the plates. Wells were drilled at similar distances around the plates using a sterilized conventional cork borer of 8 mm. Each extract concentration was aseptically placed in each well of the agar plates in a 50 mL volume. The extracts were allowed to diffuse over the agar plates for 30 min. The inoculation plates were then turned over and left to incubate for 24 h. A well-calibrated metric rule was used to measure the inhibition zone diameter for each well.[16]
Minimum inhibitory concentration (MIC)
Using the agar dilution method, the lowest inhibitory concentration of the ethanol extract on the isolates was determined. Pour plate method was used to make (6) agar plates. Six bijou bottles of nutrient agar of 19 mL each were autoclaved for this process, after which the mixture was shaken slightly for proper mixing before being poured into the Petri dishes, 1 mL of each concentration of ethanol extract was added to the bijou bottles to make up for 20 mLs in volume. The Petri dishes were marked into four equal parts, with each quadrant swabbed with the matching bacterial organism. The agar used for MIC of the C. albicans was Mueller–Hinton supplemental agar. The plates were incubated for 24 h to check for microbial growth.[16]
Determination of minimum bactericidal and minimum fungicidal concentration (MBC and MFC)
The plates from MIC were further incubated for an additional 24 h making 48 h of incubation. The lowest concentration of the extract that showed no growth after 48 h was reported as the MBC or MFC of the extract.
Gas chromatography-mass spectrometry (GC-MS) analysis
Using a Shimadzu GC-MS-QP 2010 Plus system and a gas chromatograph interfaced with a mass spectrometer system, GC-MS analysis was carried out under the following conditions. Elite: 1 capillary column made of fused silica (30 m × 0.25 mm, 1 D × L, 100% dimethyl polysiloxane). An electron ionization device with an ionization energy of 70 eV was used. With an injection volume of 2 l and a flow rate of 1 mL/min, the carrier gas was 99.99% helium gas. The ion source and injection temperatures were both set to 280°C. The oven was preheated at 110°C. Data from the National Institute of Standards and Technology collection was compared to the relative percentage amount of each component.[14]
Data analysis
After two runs of the antimicrobial susceptibility test, the results were expressed as mean ± standard error of the mean. The data generated from the study was analyzed using GraphPad Prism version 5. The difference between the values was analyzed using analysis of variance (ANOVA). P ≤ 0.05 is considered statistically significant.
RESULTS
Morphological and biochemical results of microbial isolates
From the results in Table 1, the biochemical tests on the organisms were used to confirm the presence of C. albicans, P. aeruginosa, S. aureus, E. coli, and B. subtilis.
| Microbial isolate | Cultural characteristics | Gram staining | Catalase test | Coagulase test | Indole test | Oxidase test | Lactophenol cotton blue staining |
|---|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | Greenish blue size 2–4 mm in smooth surface in Cetrimide Agar | −(ve) rod | +(ve) | −(ve) | −(ve) | +(ve) | ND |
| Escherichia coli | Colonies on eosin methylene blue agar are 2–3 mm in diameter and have a metallic green sheen when reflected light is used. | − (ve) rod | +(ve) | −(ve) | +(ve) | −(ve) | ND |
| Bacillus subtilis | Whitish convex shape in Nutrient Agar | +(ve) rod | +(ve) | −(ve) | −(ve) | −(ve) | ND |
| Staphylococcus aureus | Golden yellow 2–3 mm smooth shiny surface in Mannitol Salt Agar | +(ve) cocci | +(ve) | +(ve) | −(ve) | −(ve) | ND |
| Candida albicans | White color on SDA with a smooth and yeast-like appearance | ND | ND | ND | ND | ND | Circular bluish colonies appearing in clusters |
ND: Not determined, −ve: Negative, +ve: Positive, SDA: Saboraud dextrose agar
Antimicrobial susceptibility testing of the ethanol extract
The antimicrobial susceptibility testing results of the extracts on the microbial isolates with respect to their mean zones of inhibition are shown in Table 2. The E. coli showed the greatest zone of inhibition at 200 mg/mL with a 9.5 mm diameter, followed by 100 mg/mL with a 7 mm diameter, and the lowest zone at 6.5 mg/mL with a 1.5 mm diameter. Regarding Bacillus sp., the largest zone of inhibition measured 200 mg/mL with a 6 mm diameter was followed by 100 mg/mL with a 5 mm diameter, and the lowest was 6.5 mg/mL with a 0.5 mm diameter. The zones of inhibition for Pseudomonas sp. were determined to be 200 mg/mL with a diameter of 6 mm, 25 mg/mL with a 5.5 mm diameter, and 6.5 mg/mL with a 1 mm diameter, which was the lowest. The maximum zone of inhibition for S. aureus was discovered to be 200 mg/mL with a 6 mm diameter followed by 100 mg/mL with a 5 mm diameter, and the lowest zone was discovered to be 6.5 mg/mL with a 1.5 mm diameter. The zones of inhibition for Candida sp. were discovered to be 200 mg/mL with a 17.5 mm diameter 100 mg/mL with a 16.5 mm diameter, and 6.5 mg/mL with an 11 mm diameter, which was the lowest. With P = 0.9278, there is no significant difference in the analysis conducted horizontally between the columns when comparing them using one-way ANOVA.
| Concentration | Zone of Inhibition (mm) (X±SEM) | ||||
|---|---|---|---|---|---|
| Escherichia coli | Bacillus subtilis | Pseudomonas aeruginosa | Staphylococcus aureus | Candida sp. | |
| 200 mg/mL | 9.5±1.5 | 6±0 | 6±3 | 6±1 | 17.5±0.5 |
| 100 mg/mL | 7±1 | 5±0 | 4.5±3.5 | 5±0 | 16.5±0.5 |
| 50 mg/mL | 5±1 | 3±1 | 4.5±2.5 | 3±1 | 15±0 |
| 25 mg/mL | 3.5±0.5 | 2±1 | 5.5±0.5 | 3.5±0.5 | 14.5±0.5 |
| 12.5 mg/mL | 2.5±0.5 | 1.5±0.5 | 3.5±2.5 | 2.5±1.5 | 12±1.0 |
| 6.25 mg/mL | 1.5±0.5 | 0.5±0.5 | 1±1 | 1.5±0.5 | 11±0 |
| Ciprofloxacin (5 µg) | 22±2 | 18±4 | 15±1 | 26.5±0.5 | 0 |
| Fluconazole (10 µg) | ND | ND | ND | ND | 18±0 |
| DMSO | 0 | 0 | 0 | 0 | 0 |
The mean±SEM is used to represent the values. SEM: Standard error of the mean, DMSO: Dimethylsulfoxide, X: Mean, SEM: Standard error of mean.
MIC, MBC, and MFC of ethanol extracts on the isolates
The ethanol extract of A. indica’s MIC for S. aureus, E. coli, C. albicans, and P. aeruginosa was determined to be 12.5 mg/mL and 25 mg/mL, respectively, for Bacillus sp. as shown in Table 3.
| Isolates | MIC (mg/mL) |
MBC or MFC (mg/mL) |
|---|---|---|
| Pseudomonas aeruginosa | 12.5 | 12.5 |
| Escherichia coli | 12.5 | 100 |
| Bacillus subtilis | 25 | 0 |
| Staphylococcus aureus | 12.5 | 12.5 |
| Candida sp. | 12.5 | 12.5 |
MIC: Minimum inhibitory concentration, MFC: Minimum fungicidal concentration, MBC: Minimum bactericidal concentration
The MBC for Pseudomonas sp. is 12.5 mg/mL. For E. Coli, the MBC is 100 mg/mL. For Bacillus sp., there is no bactericidal activity as there was growth at all concentrations. For S. aureus, concentrations 200 mg/mL, 100 mg/mL, 50 mg/mL, 25 mg/mL, and 12.5 mg/mL showed the absence of S. aureus but concentration 6.25 mg/mL showed the presence of S. aureus; hence, the MBC is 12.5 mg/mL. For C. albicans, the MBC is 12.5 mg/mL.
GC-MS
In this work, the GC-MS analysis was carried out on the ethanol and n-hexane extract of the dried bark of A. indica commonly known as the Neem plant to analytically identify, quantify, and characterize the bioactive constituents of this extract.
The GC-MS analysis was carried out and the analytical results of N-Hexane and ethanol extract are shown in Tables 4 and 5, respectively. In the N-Hexane GC-MS result, it was observed that some constituents were more prominent in quantity than other constituents which made 6H-Purin-6-one,2-(dimethylamino)-1,7-dihydro the highest in terms of quantity based on the area percentage that it possesses which is 29.30% and the lowest quantity is the gamma-sitosterol occupying 1.25% in area, while from the ethanol extract, the result shows that constituent with the highest quantity occupied based on area percentage is linoelaidic acid occupying 20.45% and the lowest quantity occupied in area percentage is the 2H-1,4rbenzoxazin-3 (4H)-one with 0.19%.
| Peak | Retention time (s) | Area (%) | Compound ID | Molecular weight (g/mol) | Molecular formula |
|---|---|---|---|---|---|
| 1 | 12.582 | 3.04 | 2-undecone, 6,10-dimethyl | 198.34 | C13H26O |
| 2 | 13.425 | 9.38 | Pentadecanoic acid, 14-methyl-methyl ester | 270.4507 | C17H34O |
| 3 | 14.104 | 4.49 | Hexadecanoic acid, ethyl ester | 284.4772 | C18H36O2 |
| 4 | 15.068 | 3.77 | 9,12-octadecadienoic acid, methyl ester | 294.4721 | C19H34O |
| 5 | 15.130 | 8.52 | 9-octadecanoic acid (z) methyl ester | 294.4879 | C19H36O |
| 6 | 15.254 | 2.64 | Phytol | 294.5310 | C20H40O |
| 7 | 15.377 | 7.43 | Methyl stearate | 298.50 | C19H38O2 |
| 8 | 15.620 | 1.87 | 9,17-octadecadienal (z) | 264.4461 | C18H32O |
| 9 | 16.215 | 3.33 | 3,7,11,15-tetramethyl-2-hexadecon-1-ol | 294.5319 | C20H40O |
| 10 | 16.792 | 1.97 | 1,1-biphenyl, 2,2’,4,4’-tetrachloro | 291.9880 | C12H6Cl4 |
| 11 | 17.215 | 3.11 | 4,5,6,7-tetrafluorobenzimidazol (1,2–9) pyrazolo (3,2-C) quinazoline-3-carboxylcacid, ethylester | 240.2800 | C10H12N2O3S |
| 12 | 19.144 | 4.82 | Bis (2-ethylhexyl) phthalate | 390.5561 | C24H38O4 |
| 13 | 19.316 | 15.05 | Stigmasterol | 412.69 | C29H48O |
| 14 | 21.763 | 29.30 | 6H-Purin-6-one, 2-(dimethylamino)-1,7-dihydro | 400.0661 | C10H12N10O6 |
| 15 | 22.425 | 1.25 | Gamma-sitosferol | 414.7067 | C29H50O |
| Peak | Retention time (s) | Area (%) | Compound ID | Molecular weight (g/mol) | Molecular formula |
|---|---|---|---|---|---|
| 1 | 11.844 | 0.58 | Tetradecanoic acid | 228.3709 | C14H28O2 |
| 2 | 12.877 | 0.50 | Maltose | 342.2965 | C12H22O11 |
| 3 | 13.435 | 8.08 | Pentadecanoicacid, 14-methyl-methyl ester | 270.4507 | C17H34O2 |
| 4 | 13.806 | 0.26 | Dibutyl phthalate | 278.3435 | C16H22O4 |
| 5 | 14.139 | 16.95 | N-hexadecanoic acid | 256.4241 | C16H32O2 |
| 6 | 14.220 | 0.43 | 2-acetyl-3-nitrobenzoic acid | 209.1560 | C9H7NO5 |
| 7 | 14.258 | 0.19 | 2h-1,4rbenzoxazin-3 (4h)-on | 149.15 | C8H7NO2 |
| 8 | 14.882 | 0.90 | 1-deoxy-d-altriol | 166.17 | C6H14NO5 |
| 9 | 15.096 | 9.41 | 9,12,-octadecadienoicacid, methyl ester | 299.4721 | C19H34O |
| 10 | 15.168 | 9.78 | 7- octadecadienoicacid, methyl ester | 296.4879 | C19H36O2 |
| 11 | 15.377 | 4.91 | Methyl stearate | 298.50 | C19H38O2 |
| 12 | 15.768 | 20.45 | Linoelaidic acid | 280.4455 | C18H32O2 |
| 13 | 15.820 | 4.98 | Cis-vaccenic acid | 282.5 | C18H34O2 |
| 14 | 15.949 | 2.59 | Octadecanoic acid | 284.4772 | C18H36O2 |
| 15 | 16.849 | 1.43 | Ethanone, 1-(6-hydroxy-2-(-1-methylethenyl)-5-benzofuranyl | 216.2756 | C14H16O2 |
| 16 | 16.920 | 0.66 | 5-(1,1-ethylenedioxyethyl)-1,3 diazaazulene | 302.4 | C21H18O2 |
| 17 | 14.982 | 1.85 | 5-(1,1-ethylenedioxyethyl) 1,3-diazaazulene | 302.4 | C21H18O2 |
| 18 | 17.154 | 0.71 | Methyl-18-methylnonadecanoate | 326.5570 | C21H42O2 |
| 19 | 17.435 | 2.21 | 3-phenylthio-2h-chromen-2-one | 238.24 | C15H10O3 |
| 20 | 17.549 | 0.97 | Eicosanoic acid | 312.5304 | C20H40O2 |
| 21 | 17.882 | 0.78 | 8h-imidazo (4,5-)-2,1,3,-benzothiadiazole-7-methyl | 190.22 | C8H6N4S |
| 22 | 18.801 | 1.16 | Glycerol-1-palmitate | 330.5026 | C19H38O |
| 23 | 20.968 | 6.50 | 9-octadecenoic acid (z)-2-hydroxy-1-(hydroxymethyl) ethyl ester | 354.5240 | C21H40O4 |
| 24 | 23.358 | 3.72 | Vitamin E | 430.7041 | C29H50O2 |
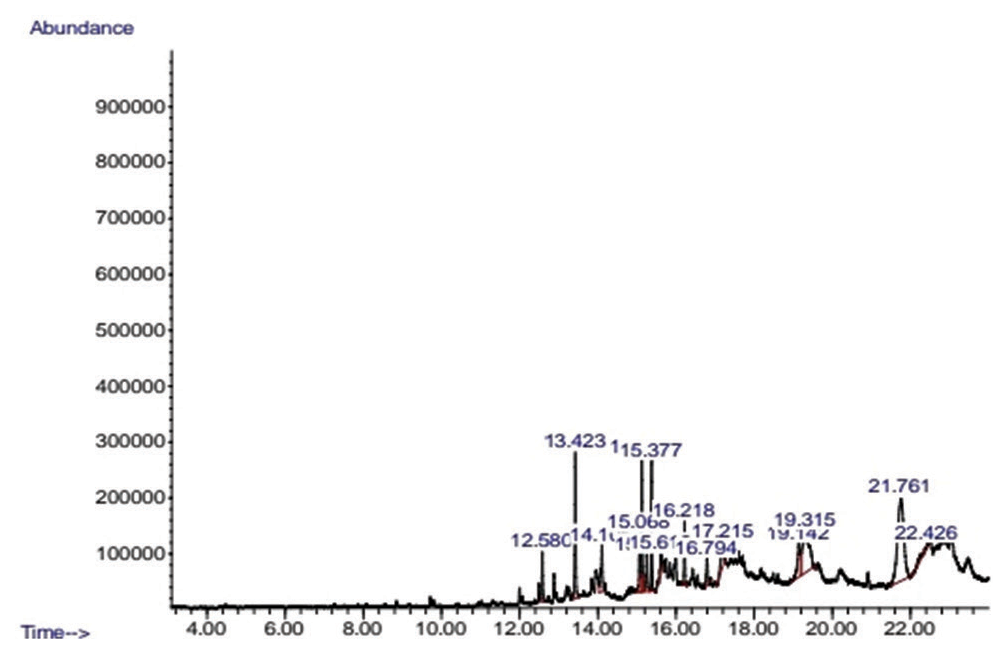
- Chromatogram of n-hexane extract of neem bark.
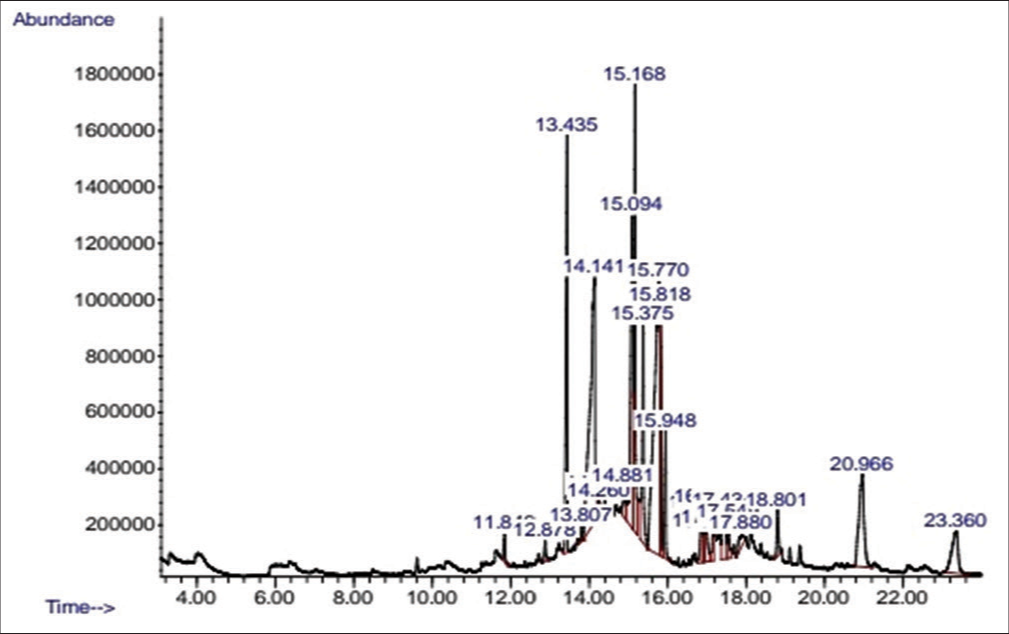
- Chromatogram of ethanol extract of neem bark.
DISCUSSION
The A. indica plant is a natural medication store. Antibacterial activity was reported in all concentrations of A. indica bark extract, with antibacterial activity against S. aureus seen at higher doses of >6.12 mg/mL. At all doses except 6.12 mg/mL, the bark of A. indica demonstrated a zone of clearance against P. aeruginosa. Higher doses produced a zone of inhibition against S. aureus. The method by which bacteria typically withstand the action of antimicrobial drugs is unknown and controversial. The findings of this study support Okemo et al.’s claim that A. indica components might be utilized safely as chemotherapeutic agents at predefined quantities.[17] The antibacterial action of A. indica extracts utilized in this investigation is demonstrated in Tables 2 and 3 used in the interpretation of the findings. In all instances, ethanol extracts were the most effective against S. aureus. The bactericidal activity of all extracts used in this study increased with increasing extract concentration, suggesting that on plates containing extract with a low dilution factor, the inhibitory zone was bigger. This was also noted by Esimone et al., who opined that varying concentrations of neem plant extract are inhibitory to bacterial growth.[18] This is in line with the investigation’s results, which showed that the diameter of inhibitory zones increased in proportion to an increase in extract concentration.[19] S. aureus and P. aeruginosa were unaffected by low concentrations of neem extract; however, when the dilution level dropped, the effect of the extract concentration increased. These studies’ findings are in line with those of Alorzohairy, who stated that traditional Indian medicine has utilized various neem tree parts, such as the bark (A. indica), for their purported bioactive, therapeutic, and preventive qualities.[20] The results of Wylie and Merrell’s study, which found that a variety of A. indica components, including seeds, barks, and leaves produce extracts with moderate to strong antimicrobial activity against a range of pathogens, including S. aureus, E. faecalis, P. aeruginosa, E. coli, Salmonella. Typhi, Shigella boydii, B. subtilis, Candida tropicalis, K. pneumoniae, and Streptococcus agalactiae.[21] The reports of this work are also in line with the work of Singaravelu et al. which reported the antimicrobial effect of A. indica bark extracts on P. aeruginosa and Pseudomonas mirabilis.[22] The results of this work are also consistent with the work of other authors, who reported the antimicrobial effect of A. indica extracts on S. aureus, P. aeruginosa, and E. coli.[23]
Some compounds identified in the A. indica have been previously reported to have useful characteristics. Compounds, like Linoelaidic acid found in the ethanol extract, have been previously reported to possess antibacterial effect on gram-positive isolates as can be seen in the work of Dilika et al.[24] Dewi et al.[25] reported antimicrobial effects on S. aureus and B. subtilis. Desbois and Smith[26] reported antibacterial activity in pathogenic bacteria. Stigmasterol molecules from the n-hexane extract have been reported to have antimicrobial effect as can be seen in the report of Yusuf et al. on S. aureus, E. coli, and C. albicans.[27] Yohanna et al.[28] reported the antimicrobial effect of stigmastrol on bacteria isolates. Hexadecanoic acid, also known as palmitic acid, was present in both extracts. It has also been previously reported to possess an antibacterial effect.[29,30]
CONCLUSION
This research found that an ethanol extract of A. indica bark has antimicrobial properties against P. aeruginosa, S. aureus, E. coli, C. albicans, and B. subtilis. The extracts contained some bioactive compounds. Some of the compounds identified including Linoleic acid, Stigmasterol, and Hexadecanoic acid have previously been identified as antimicrobial compounds. The other compounds can potentially contribute to the antimicrobial effect of the extracts. More research is needed to determine the individual compounds that actually have antimicrobial properties.
Acknowledgments
We acknowledge the invaluable assistance of individuals involved in this research work especially Prof. E.E. Obaseki-Ebor, and the technologists in the Department of Pharmaceutical Microbiology and Biotechnology, Madonna University Elele, Rivers State.
Authors’ contributions
Oluchi Judith Osuala: Conceptualization, Literature collection and curation, writing–original draft.
Samuel Igwe: Literature collection and curation. Chinyere Constance Ezemba: Literature collection and curation. Chukwuma Chukwuemeka Chukwuma: Literature collection and curation. Angus Nnamdi Oli: Literature collection and curation, writing–original draft, review and editing.
Ethical approval
The Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent was not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
None.
References
- Bacterial infections: Overview In: International encyclopedia of public health. United States: Britannica. Inc.; 2008. p. :273-82.
- [CrossRef] [Google Scholar]
- Human gut microbiota: Repertoire and variations. Front Cell Infect Microbiol. 2012;2:136. doi: 10.3389/fcimb.2012.00136
- [CrossRef] [PubMed] [Google Scholar]
- Secondary metabolites of endophytic fungi from Newbouldia laevis and Cassia tora leaves: Prospecting for new antimicrobial agents. Recent Pat Antiinfect Drug Discov. 2021;16:50-62. doi: 10.2174/1574891X15999201222152646
- [CrossRef] [PubMed] [Google Scholar]
- Winning the war against multi-drug resistant diarrhoeagenic bacteria. Microorganisms. 2019;7:197. doi: 10.3390/microorganisms7070197
- [CrossRef] [PubMed] [Google Scholar]
- Screening of Tanzanian medicinal plants for anti-Candida activity. BMC Complement Altern Med. 2006;6:11. doi: 10.1186/1472-6882-6-11
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial activity of southern African medicinal plants with dermatological relevance: From an ethnopharmacological screening approach to combination studies and the isolation of a bioactive compound. J Ethnopharmacol. 2013;148:45-55. doi: 10.1016/j.jep.2013.03.056
- [CrossRef] [PubMed] [Google Scholar]
- Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel). 2013;6:1451-1474. doi: 10.3390/ph6121451
- [CrossRef] [PubMed] [Google Scholar]
- Combinations of Alchornea cordifolia, Cassytha filiformis and Pterocarpus santalinoides in diarrhoegenic bacterial infections. BMC Res Notes. 2019;12:649. doi: 10.1186/s13104-019-4687-02
- [CrossRef] [PubMed] [Google Scholar]
- The role and place of medicinal plants in the strategies for disease prevention. Afr J Tradit Complement Altern Med. 2013;10:210-229. doi: 10.4314/ajtcam.v10i5.2
- [CrossRef] [PubMed] [Google Scholar]
- Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper Geneva: WHO; 2019.
- [Google Scholar]
- Tackling Drug-Resistant Infections Globally: Final Report and Recommendations, Review on Antimicrobial Resistance In: Wellcome Trust and HM Government. 2016. p. :1-84.
- [Google Scholar]
- Antimicrobial resistance: A one health perspective. Microbiol Spectr. 2018;6:1-26. doi: 10.1128/microbiolspec.ARBA-0009-2017
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial activities of green algae on microbial isolates. J Adv Microbiol Res. 2020;1:37-43.
- [Google Scholar]
- Antimicrobial effect of cinnamon bark extracts on microbial isolates. J Adv Microbiol Res. 2020;1:42-50. doi: 10.2139/ssrn.4450001
- [CrossRef] [Google Scholar]
- District laboratory practice in tropical countries (2nd ed). Cambridge, United Kingdom: University Press; 2010.
- [Google Scholar]
- EUCAST definitive document E.Def 1.2, May 2000: Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin Microbiol Infect. 2000;6:503-8. doi: 10.1046/j.1469-0691.2000.00149.x
- [CrossRef] [PubMed] [Google Scholar]
- The kill kinetics of Azadirachta indica A. JUSS. (Meliaceae) extracts on Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans. Afr J Sci Technol. 2001;2:113-118.
- [Google Scholar]
- In vitro antimicrobial evaluation of lozenges containing extract of garlic and ginger. Int J Health Res. 2010;3:105-110. doi: 10.4314/ijhr.v3i2.70274
- [CrossRef] [Google Scholar]
- Octopamine neuromodulation regulates gr32a-linked aggression and courtship pathways in Drosophila males. PLoS Genet. 2014;10:e1004356.
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Altern Med. 2016;2016:7382506. doi: 10.1155/2016/7382506
- [CrossRef] [PubMed] [Google Scholar]
- The antimicrobial potential of the neem tree Azadirachta indica. Front Pharmacol. 2022;13:891535.
- [CrossRef] [PubMed] [Google Scholar]
- Effect of Azadirachta indica crude bark extracts concentrations against gram-positive and gram-negative bacterial pathogens. J Pharm Bioallied Sci. 2019;11:33-37. doi: 10.4103/jpbs.JPBS_150_18
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial activity of Azadirachta indica (Neem) leaf extract on some bacteria. Int J Curr Microbiol Applied Sci. 2019;8:431-437.
- [CrossRef] [Google Scholar]
- Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia. 2000;71:450-452. doi: 10.1016/s0367-326x(00)00150-7
- [CrossRef] [PubMed] [Google Scholar]
- Linoleic acid, α-linolenic acid, and monolinolenins as antibacterial substances in the heat-processed soybean fermented with Rhizopus oligosporus. Biosci Biotechnol Biochem. 2020;84:1285-1290. doi: 10.1080/09168451.2020.1731299
- [CrossRef] [PubMed] [Google Scholar]
- Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629-1642. doi: 10.1007/s00253-009-2355-3
- [CrossRef] [PubMed] [Google Scholar]
- Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J Med Plants Econ Dev. 2018;2:a38. doi: 10.4102/jomped.v2i1.38
- [CrossRef] [Google Scholar]
- Antibacterial activity, antioxidant potential and stigmasterol isolation from Laggera aurita Linn (Asteraceae) Int J Biochem Res Rev. 2021;30:24-39. doi: 10.9734/ijbcrr/2021/v30i930288
- [CrossRef] [Google Scholar]
- Antibacterial activities of hexadecanoic acid methyl ester and green-synthesized silver nanoparticles against multidrug-resistant bacteria. J. Basic Microbiol. 2021;61:557-568. doi: 10.1002/jobm.202100061
- [CrossRef] [PubMed] [Google Scholar]
- Chemical profiling, antibacterial and antiparasitic studies of Imperata cylindrica. J Appl Pharm Sci. 2019;9:117-121. doi: 10.7324/JAPS.2019.91216
- [CrossRef] [Google Scholar]







