Translate this page into:
Factor Xa inhibitors versus warfarin in severely obese patients with venous thromboembolism or atrial fibrillation
-
Received: ,
Accepted: ,
How to cite this article: Tierce HE, Lusk KA, Kitten AK, et al. Factor Xa inhibitors versus warfarin in severely obese patients with venous thromboembolism or atrial fibrillation. Am J Pharmacother Pharm Sci 2024:3.
Abstract
Objectives:
Clinical data for the safety and efficacy of Factor Xa (FXa) inhibitors in severely obese patients is limited. Additional information is needed to assess the risks and benefits of using FXa inhibitors in this patient population.
Materials and Methods:
A single-center and retrospective chart review was conducted in severely obese patients (body mass index [BMI] >40 kg/m2 or weight >120 kg), who received rivaroxaban, apixaban or warfarin for venous thromboembolism (VTE) treatment or prevention of stroke and systemic embolism in non-valvular atrial fibrillation (AF). The primary endpoint was treatment failure, defined as recurrent VTE in VTE treatment or stroke, transient ischemic attack or systemic embolism in AF within one year of anticoagulation initiation. Secondary endpoints included a composite of major bleeding rates and clinically relevant non-major bleeding (CRNMB) rates.
Results:
Seventy-three patients were included in the final analysis consisting of 43 patients in the FXa inhibitor arm (apixaban [n = 33] and rivaroxaban [n = 10]) and 30 patients in the warfarin arm. The rate of treatment failure was similar between the FXa inhibitor and warfarin groups (4.7% vs. 6.7%; P = 0.814). Although not statistically significant, major bleeding and CRNMB occurred less frequently in patients receiving FXa inhibitors compared to warfarin (9.3% vs. 23.3%; P = 0.182). Subgroup analysis in patients with BMI >50 kg/m2 or weight >150 kg demonstrated similar efficacy and safety outcomes. The use of non-recommended dosing was more common in the apixaban group compared to the rivaroxaban group.
Conclusion:
FXa inhibitors (apixaban and rivaroxaban) appear to have similar efficacy and safety compared to warfarin in severely obese patients. Larger prospective studies are needed to confirm these results.
Keywords
Obesity
Direct oral anticoagulant
Warfarin
Venous thromboembolism
Atrial fibrillation
INTRODUCTION
Obesity represents a significant issue among Americans, as nearly 42% of adults are classified as obese, and 9% of adults are categorized as severely obese.[1] High total body weight and high body mass index (BMI) are associated with an increased risk of venous thromboembolism (VTE) as well as an increased incidence of atrial fibrillation (AF), although the impact of BMI on stroke in AF is controversial.[2-4] Current guidelines from the American College of Chest Physicians (CHEST) and the American Society of Hematology for acute VTE management recommend Factor Xa (FXa) inhibitors over warfarin.[5,6] Similarly, current guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend FXa inhibitors over warfarin for the prevention of stroke and systemic embolism in nonvalvular AF.[7] Compared to warfarin, FXa inhibitors are associated with reduced monitoring requirements, fewer drug/food interactions, and rapid onset of action.[8]
Clinical data for the safety and efficacy of FXa inhibitors in patients with BMI >40 kg/m2 or weight >120 kg are limited.[9] Phase 3 clinical trials that led to the approval of FXa inhibitors (rivaroxaban, apixaban, and edoxaban) in patients with VTE or AF included relatively few obese subjects ranging from 12% to 34% of the total population. Subgroup analyses suggested weight did not significantly impact FXa inhibitor’s efficacy and safety. However, clinical outcomes in extremely obese patients (BMI >40 kg/m2) were not reported, as these analyses primarily used a BMI cutoff of ≥35kg/m2 or weight >100 kg. In addition, results from pharmacokinetic (PK) studies indicated apixaban and dabigatran concentrations were affected by weight raising concerns for possible underdosing in severely obese patients.[10,11] Alternatively, a PK study with rivaroxaban found weight did not impact maximum blood concentrations, the area under the concentration-time curve or FXa activity.[12] In 2016, the International Society of Thrombosis and Hemostasis (ISTH) released guidance that suggested direct oral anticoagulants should not be used for AF or VTE treatment in patients with BMI >40 kg/m2 or weight >120 kg due to the lack of clinical outcome data.[9]
Since the ISTH Statement was released, several small, mostly single-center, and retrospective studies have explored the use of FXa inhibitors in severely obese patients with VTE and non-valvular AF.[13-22] Meta-analyses and systemic reviews conducted with these studies along with post hoc analyses from phase 3 clinical trials indicate FXa inhibitors have similar efficacy outcomes compared to warfarin including similar rates of VTE recurrence in the VTE population and stroke/systemic embolism in the AF population.[23-25] Major bleeding rates also appear to be similar or reduced in obese patients receiving FXa inhibitors for VTE and AF compared to warfarin. In 2021, the ISTH provided updated recommendations suggesting standard doses of apixaban and rivaroxaban could be used for the treatment of VTE regardless of obesity but did not provide recommendations for FXa inhibitor use in obese patients with AF.[26] In addition, the authors of the ISTH Statement noted that clinical data was limited for the safety and efficacy of FXa inhibitors in obese patients with BMI >50 kg/m2 and weight >150 kg in the treatment of VTE.
As FXa inhibitors continue to be prescribed with increasing frequency over warfarin, more data is needed to further define the risks and benefits of FXa inhibitors in severely obese patients.[27] The purpose of this retrospective chart review was to determine the safety and efficacy of FXa inhibitors, specifically rivaroxaban and apixaban, compared to warfarin in patients with VTE or non-valvular AF and BMI >40 kg/m2 or weight >120 kg. In addition to the evaluation of the safety and efficacy of FXa inhibitors, this study assessed the dosing regimens used. The safety and efficacy of FXa inhibitor’s use in patients with BMI >50 kg/m2 or weight >150 kg were also evaluated.
MATERIALS AND METHODS
Study design
A retrospective chart review was conducted to assess the safety and effectiveness of FXa inhibitors in severely obese patients hospitalized for non-valvular AF or VTE. The data was collected from patients, who visited one major academic medical center from January 1, 2016, to January 1, 2020. University of Texas Health Science Center at San Antonio Institutional Review Board approved this study, which was determined to be exempt human subjects research (Protocol Number HSC20200913E). Consequently, Health Insurance Portability and Accountability Act (HIPAA) authorization was not required. The Research Electronic Data Capture system, a HIPAA-compliant web-based application, was used to securely collect de-identified data of patients who met inclusion criteria.[28,29]
Patient population selection
International classification of diseases (ICD)-9 and ICD-10 codes were used to initially identify patients for possible inclusion in the study. Electronic medical records were then utilized to collect clinical outcome data up to 1 year after anticoagulation initiation. Inclusion criteria were defined as BMI >40 kg/m2 or weight >120 kg at the time of anticoagulation initiation, diagnosis of acute VTE or non-valvular AF during hospitalization, and prescribed rivaroxaban, apixaban, or warfarin. Due to lack of clinical data and institutional formulary restrictions, patients who received other direct oral anticoagulants (dabigatran, edoxaban, and betrixaban) were not included in this study. Patients were excluded if they were <18 years old, pregnant, breastfeeding, received warfarin, or FXa inhibitors before admission, required anticoagulation for combined VTE and AF treatment, reported the presence of bioprosthetic or mechanical heart valve, and used FXa inhibitors for VTE prophylaxis after total hip or knee arthroplasty, active malignancy or acute decompensated cirrhosis. Baseline characteristics were collected on the day of anticoagulation initiation including medical history, tobacco use, and concomitant medications that may increase clotting or bleeding risk. CHA2DS2-VASc and HAS-BLED (Hypertension, Abnormal renal/hepatic function, Stroke - Bleeding, Labile INR, Elderly, and Drugs/alcohol excess) scores were calculated for all AF patients.
Outcomes
The primary outcome was the incidence of treatment failure within 1 year of anticoagulation initiation. Treatment failure was defined as recurrent VTE within the VTE group and incidence of ischemic stroke, transient ischemic attack (TIA), or systemic embolism within the AF group. Recurrent VTE was determined by new or worsening deep vein thrombosis or pulmonary embolism as seen on imaging or with high suspicion in patients with previous VTE. Ischemic stroke, TIA or systemic embolism was determined by the presence on diagnostic imaging or high suspicion in patients with non-valvular AF. Secondary outcomes include a composite of major bleeding and clinically relevant non-major bleeding (CRNMB) rates as defined by ISTH criteria [Supplemental Table 1].[30]
In addition, anticoagulant dosing at therapy initiation was evaluated and separated into recommended dosing versus non-recommended dosing as defined by the package insert for each specific agent [Supplemental Table 2].[31,32] Recommended dosing was defined as the appropriate use of standard dosing or adjusted dosing for renal clearance. Non-recommended dosing was defined as the use of inappropriate dosing based on indication or inappropriate duration of lead in phase for patients in the VTE group.
Statistical analysis
The Chi-square and Fisher’s exact, Wilcoxon Rank sum, and Student’s t-tests were used to analyze nominal data, non-normally distributed numeric data, and normally distributed numeric data, respectively. Statistically significant results were identified using an alpha level of 0.05. JMP 16.0 software (SAS Institute, Cary, NC) was used for statistical analysis.
RESULTS
A total of 840 patients were screened, and 73 patients were included in the final analysis [Figure 1]. The most common reason for exclusion was not meeting inclusion criteria, specifically weight and BMI requirements (i.e., BMI <40 kg/m2 or weight <120 kg) followed by receiving warfarin or an FXa inhibitor before admission. Baseline characteristics were generally balanced between the groups [Table 1]. Patients with AF were more likely to receive an FXa inhibitor versus warfarin (53% vs. 23%, P = 0.010). In the AF group, apixaban was the most common anticoagulant, followed by warfarin and rivaroxaban (67% vs. 23% vs. 10%). The most used anticoagulant in the VTE group was warfarin, followed by apixaban and rivaroxaban (54% vs. 29% vs. 17%). Medications that increase bleeding risk were similar between groups, except for aspirin, which was more common in the FXa inhibitor group (49% vs. 23%, P = 0.028). The FXa inhibitor group also reported significantly higher HAS-BLED scores (1 [1–2] vs. 0 [0–1], P = 0.032). Of note, the warfarin group reported higher weight and BMI at anticoagulation initiation, although this difference was not statistically significant.
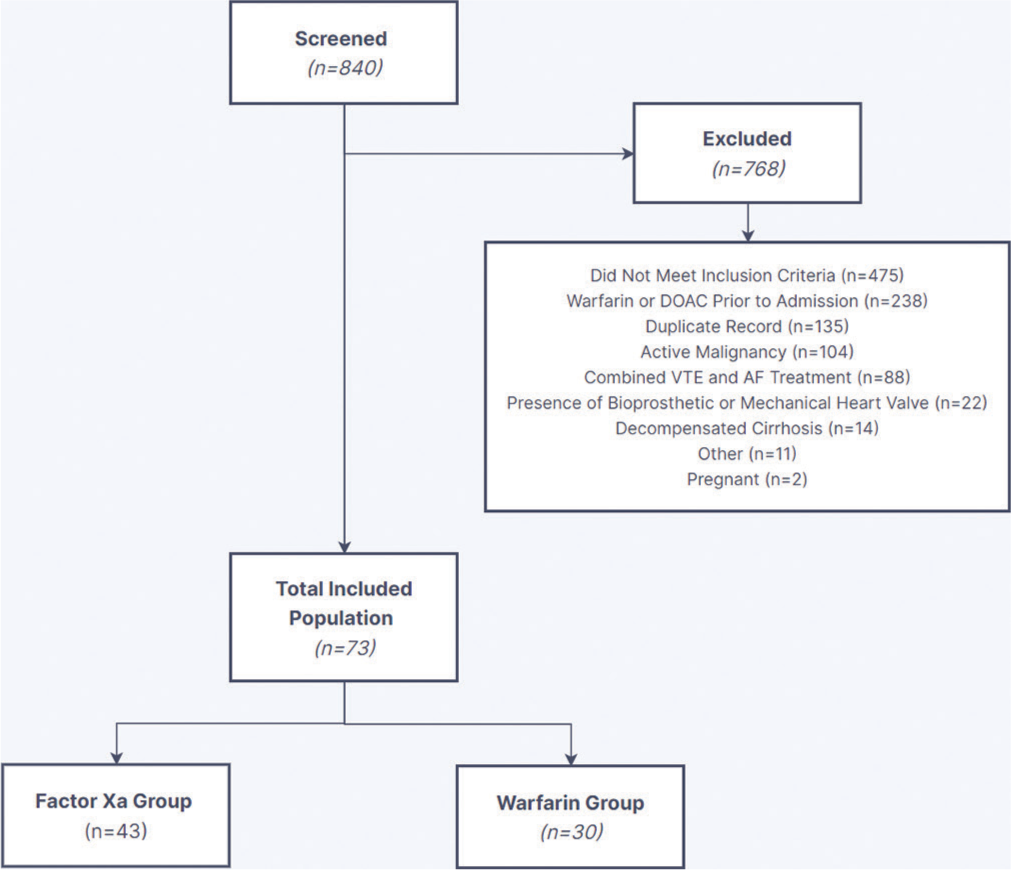
- Data collection process. DOAC: Direct oral anticoagulants, VTE: Venous thromboembolism, AF: Atrial fibrillation.
| Baseline characteristic | FXa inhibitor (n=43) | Warfarin (n=30) | P-value |
|---|---|---|---|
| Age, years, median (IQR) | 53 (42–57) | 49 (38–61) | 0.526 |
| Male, n(%) | 30 (74) | 18 (60) | 0.279 |
| Race, n(%) | 0.503 | ||
| Caucasian | 36 (84) | 27 (90) | - |
| African American | 7 (16) | 3 (10) | - |
| Ethnicity, n(%) | 0.874 | ||
| Hispanic | 18 (42) | 12 (40) | - |
| Non-Hispanic | 25 (58) | 18 (60) | - |
| Choice of FXa inhibitor, n(%) | |||
| Apixaban | 33 (77) | - | - |
| Rivaroxaban | 10 (23) | ||
| Weight, kg, median (IQR) | 129 (122–151) | 140 (124–174) | 0.107 |
| BMI, kg/m2, median (IQR) | 44 (41–50) | 50 (43–59) | 0.077 |
| AF, n(%) | 23 (53) | 7 (23) | 0.010 |
| VTE, n(%) | 20 (47) | 23 (77) | 0.413 |
| DVT | 1 (2) | 0 | - |
| PE | 11 (26) | 15 (50) | - |
| DVT and PE | 8 (19) | 8 (27) | - |
| Medical history, n(%) | |||
| Prior VTE | 1 (2) | 2 (7) | 0.814 |
| Genetic clotting disorder | 1 (2) | 3 (10) | 0.299 |
| Diabetes mellitus | 20 (47) | 12 (40) | 0.455 |
| Chronic kidney disease | 9 (21) | 6 (20) | 0.741 |
| Heart failure | 16 (37) | 8 (27) | 0.345 |
| Prior stroke/TIA | 3 (7) | 3 (10) | 0.685 |
| Liver disease | 3 (7) | 0 | 0.264 |
| Major surgery | 4 (9) | 4 (13) | 0.709 |
| Tobacco use | 9 (21) | 3 (10) | 0.215 |
| Laboratory values | |||
| Serum creatinine, mg/dL, median (IQR) | 1.0 (0.8–1.2) | 0.9 (0.7–1.3) | 0.355 |
| Hemoglobin, g/dL, mean±SD | 12.7±2.4 | 11.7±2.4 | 0.087 |
| Platelets, 103/mm3, median (IQR) | 213 (158–267) | 232 (164–264) | 0.784 |
| CHA2DS2-VASc score, median (IQR) | 2 (2–3) | 3 (2–3) | 0.277 |
| HAS-BLED score, median (IQR) | 1 (1–2) | 0 (0–1) | 0.032 |
| Concomitant medications, n(%) | |||
| Aspirin | 21 (49) | 7 (23) | 0.028 |
| P2Y12 Inhibitor | 1 (2) | 0 | 0.400 |
| NSAID | 3 (7) | 2 (7) | 0.668 |
FXa: Factor Xa inhibitor, AF: Atrial fibrillation, VTE: Venous thromboembolism, DVT: Deep vein thrombosis, PE: Pulmonary embolism, TIA: Transient ischemic attack, NSAID: Non-steroidal anti-inflammatory drug, SD: Standard deviation, IQR: Interquartile range, BMI: Body mass index
The primary outcome of treatment failure was similar between the FXa inhibitor and warfarin groups (5% vs. 7%, P = 0.814; [Table 2]). Although there was no statistically significant difference in the rates of major bleeding or CRNMB, there was a trend toward decreased bleeding rates in the FXa inhibitor group compared to the warfarin group (9% vs. 23%, P = 0.182). This trend appears to have been driven by major bleeding rates, specifically due to a drop in hemoglobin ≥2 g/dL in the warfarin group (13% vs. 2%, P = 0.152).
| Outcome | FXa inhibitor (n=43) | Warfarin (n=30) | P-value |
|---|---|---|---|
| Treatment failure, n(%) | 2 (5) | 2 (7) | 0.814 |
| VTE Recurrence | 2 (5) | 2 (7) | - |
| Stroke, TIA, systemic embolism | 0 | 0 | - |
| Major bleeding or CRNMB, n (%) | 4 (9) | 7 (23) | 0.182 |
| Drop in hemoglobin ≥2 g/dL | 1 (2) | 4 (13) | 0.152 |
| Transfusion of ≥2 units of RBC or whole blood | 0 | 3 (10) | 0.065 |
| Symptomatic bleeding in critical area or organ | 0 | 0 | - |
| Bleeding leading to death | 0 | 0 | - |
| CRNMB | 3 (7) | 3 (10) | 0.685 |
FXa: Factor Xa inhibitor, VTE: Venous thromboembolism, TIA: Transient ischemic attack, CRNMB: Clinically relevant non-major bleeding, RBC: Red blood cells
Patients receiving apixaban reported higher rates of non-recommended dosing compared to rivaroxaban (3/33 [9%] vs. 0/10 [0%]). All non-recommended dosing in the apixaban group was due to decreased maintenance dose (1/33 [3%]) and missing or shortened lead in phase for VTE treatment (2/33 [6%]). Treatment failure did not occur in any patient who received a non-recommended dosing regimen. In a subgroup analysis of 32 patients with BMI >50 kg/m2 or weight >150 kg, there was no difference in treatment failure between the FXa inhibitor and warfarin groups (0/15 [0%] vs. 1/17 [6%], P = 0.611; [Supplemental Table 3]). Similarly, rates of major bleeding or CRNMB did not differ between groups in the patients with BMI >50 kg/m2 or weight >150 kg, but the FXa inhibitor group experienced numerically lower bleeding rates compared to the warfarin group (13% vs. 24%, P = 0.596).
DISCUSSION
This retrospective chart review found patients with severe obesity (BMI >40 kg/m2 or weight >120 kg) experienced similar rates of treatment failure and bleeding when prescribed an FXa inhibitor (rivaroxaban and apixaban) or warfarin for the treatment of VTE or stroke/systemic embolism prevention in non-valvular AF. Although the rate of non-recommended dosing was higher in the apixaban group, recommended dosing was used in most patients. Despite the ISTH guidance statement in 2016, the FXa inhibitor group was larger than the warfarin group, signaling that FXa inhibitors have continued to be used in the obese population.
Based on the results obtained in this chart review, apixaban or rivaroxaban are appropriate treatment options in severely obese patients (BMI >40 kg/m2 or weight >120 kg) at standard doses for VTE or AF. These results correlate with previous studies that compared FXa inhibitors versus warfarin in patients with higher body weights. Kushnir et al. conducted one of the largest single-center retrospective studies in severely obese adults (BMI >40 kg/m2) who were prescribed apixaban, rivaroxaban, or warfarin for VTE or AF (n = 795).[13] Efficacy outcomes were similar between FXa inhibitors and warfarin specifically VTE recurrence in the VTE group and stroke in the AF group (P = 0.74 and P = 0.71, respectively). Clinically, relevant major and non-major bleeding rates were also similar in both VTE and AF populations (P = 0.45 and P = 0.16, respectively). When analyzed by BMI, a multicenter retrospective cohort study found efficacy and safety of FXa inhibitors were maintained as BMI increased.[14] In a recent meta-analysis of retrospective studies and post hoc analysis of phase 3 clinical trials, FXa inhibitors were associated with significant reductions in the risk of major bleeding across the spectrum of weight (P = 0.0007), with no significant difference in VTE recurrence and stroke (P = 0.96).[23] Notably, the dosing regimens in the FXa inhibitor group were often not addressed which could influence clinical outcomes. In a reply to a letter to the editor, Kushnir noted that standard dosing was used in most patients (94% in the rivaroxaban group, 89% in the apixaban group).[33] Quan et al. found suboptimal dosing was present in 14.8% of obese patients (weight >120 kg) receiving an FXa inhibitor for VTE treatment.[34] In the present study, recommended dosing was used in over 90% of patients receiving an FXa inhibitor, which is higher than previously reported.[34]
Studies conducted in patients with solely VTE or AF have largely shown a similar or decreased risk of recurrent VTE, stroke, and bleeding with FXa inhibitors versus warfarin.[15,16] However, one study found that AF patients with a higher BMI were more likely to experience worse outcomes with direct oral anticoagulants (specifically dabigatran, rivaroxaban, and apixaban) including shorter time to stroke/systemic embolism (P = 0.043) and major bleeding (P < 0.001).[17] About 55% of the study population received dabigatran, which had previously been shown to be affected by weight in a PK study.[11,17] On the other hand, another study found no difference in stroke, mortality, or clinically relevant bleeding in patients who weighed >120 kg versus ≤120 kg who received dabigatran.[18] In meta-analyses of direct oral anticoagulants in patients with VTE or AF alone, efficacy and safety outcomes were similar between direct oral anticoagulants versus warfarin in severely obese patients.[24,25] Specifically, apixaban and rivaroxaban have been shown to be safe and efficacious in patients with higher body weights in retrospective studies with each individual agent and is further demonstrated in the results of this study.[19-22]
Although there is emerging data supporting the use of direct oral anticoagulants in patients with BMI >40 kg/m2 or weight >120 kg, clinical outcome data in patients with BMI > 50 kg/m2 or weight > 150 kg is even more limited in patients with AF or VTE. In fact, some authors recommend using warfarin and avoiding FXa inhibitors in patients with BMI >50 kg/m2 or weight > 150 kg in the treatment of AF due to a lack of clinical data.[35] One single-center retrospective study analyzed apixaban and rivaroxaban use in patients with AF and BMI > 50 kg/m2 (n = 595).[36] In this study, ischemic stroke and bleeding were not significantly different when patients with a BMI > 50 kg/m2 were compared to patients with a BMI of 18–30 kg/m2 (P = 0.544 for ischemic stroke). Similar results were found in another retrospective chart review in obese patients with weight ≥ 150 kg or BMI ≥ 50 kg/m2 who were diagnosed with VTE or AF.[37] Of note, a recent multidisciplinary expert panel recommended against using weight as a deciding factor for the choice of anticoagulation in patients with VTE but did acknowledge the lack of data in patients with extreme obesity.[38] Our study provides additional data in this patient population demonstrating that FXa inhibitors continue to be safe and efficacious even with higher weights especially in patients with VTE as shown in the subgroup analysis of patients with BMI > 50 kg/m2 or weight > 150 kg.
While this study does have several strengths, it is not without limitations. The retrospective design limited the data to information found in the electronic medical record, and data was unable to be collected if a patient visited an outside institution. Adherence rates for all medications and time in the therapeutic range for warfarin were unable to be assessed. In terms of baseline characteristics, the FXa inhibitor group reported significantly higher rates of aspirin use and higher median HAS-BLED scores, but this did not appear to affect bleeding rates. Weight at anticoagulation initiation was used which may change throughout the patient’s treatment course. Therapeutic drug monitoring of FXa inhibitors was also unavailable, although there is limited data on the utility of anti-FXa levels.[39,40] A significant number of patients screened were not included due to not meeting weight or BMI requirements. This was most likely due to inaccuracies in ICD coding that were corrected once the individual’s chart was reviewed. Finally, this study included a small number of patients with a low event rate. Thus, the results are potentially underpowered to assess the significance of the primary outcome due to the risk of type II error. Until large, randomized control trial data is available; this study provides additional data supporting the use of FXa inhibitors in severely obese patients which will be used to encourage further study.
CONCLUSION
Our study found that rivaroxaban and apixaban were associated with similar rates of VTE recurrence, stroke, and bleeding compared to warfarin in severely obese patients for the treatment of VTE and stroke or systemic embolism in non-valvular AF. Based on these results, rivaroxaban and apixaban appear to be safe and effective for obese patients, including those with BMI >40 kg/m2 or weight >120 kg. However, large prospective studies are needed to confirm these results in severely obese patients.
Ethical approval
The research/study approved by the Institutional Review Board at University of Texas Health Science Center at San Antonio, number HSC20200913E, dated 12/18/2020.
Declaration of patient consent
Patient’s consent was not required as the study was retrospective.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
None.
References
- Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;360:1-8.
- [Google Scholar]
- Cardiovascular risk factors and venous thromboembolism: A meta-analysis. Circulation. 2008;117:93-102. doi: 10.1161/CIRCULATIONAHA.107.709204
- [CrossRef] [PubMed] [Google Scholar]
- Obesity as a risk factor for the progression of paroxysmal to permanent atrial fibrillation: A longitudinal cohort study of 21 years. Eur Heart J. 2008;29:2227-2233. doi: 10.1093/eurheartj/ehn324
- [CrossRef] [PubMed] [Google Scholar]
- Association of body mass index with clinical outcomes in patients with atrial fibrillation: A report from the FANTASIIA registry. J Am Heart Assoc. 2020;9:e013789. doi: 10.1161/JAHA.119.013789
- [CrossRef] [PubMed] [Google Scholar]
- Antithrombotic therapy for VTE disease: Second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.056
- [CrossRef] [PubMed] [Google Scholar]
- American Society of Hematology 2020 guidelines for management of venous thromboembolism: Treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4:4693-4738. doi: 10.1182/bloodadvances.2020001830
- [CrossRef] [PubMed] [Google Scholar]
- 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104-132. doi: 10.1016/j.jacc.2019.01.011
- [CrossRef] [Google Scholar]
- Pros and cons of new oral anticoagulants. Hematology Am Soc Hematol Educ Program. 2013;2013:464-470. doi: 10.1182/asheducation-2013.1.464
- [CrossRef] [PubMed] [Google Scholar]
- Use of the direct oral anticoagulants in obese patients: Guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
- [CrossRef] [PubMed] [Google Scholar]
- Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. J Clin Pharmacol. 2013;76:908-916. doi: 10.1111/bcp.12114
- [CrossRef] [PubMed] [Google Scholar]
- The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: The RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy) J Am Coll Cardiol. 2014;63:321-328. doi: 10.1016/j.jacc.2013.07.104
- [CrossRef] [PubMed] [Google Scholar]
- Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol. 2007;47:218-226. doi: 10.1177/0091270006296058
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: A single-centre, retrospective analysis of chart data. Lancet Haematol. 2019;6:e359-e365. doi: 10.1016/S2352-3026(19)30086-9
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of safety and efficacy outcomes of direct oral anticoagulants versus warfarin in normal and extreme body weights for the treatment of atrial fibrillation or venous thromboembolism. J Thromb Thrombolysis. 2022;54:276-286. doi: 10.1007/s11239-022-02668-8
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness and safety of direct oral anticoagulants versus warfarin in obese patients with acute venous thromboembolism. Pharmacotherapy. 2020;40:204-210. doi: 10.1002/phar.2369
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
- [CrossRef] [PubMed] [Google Scholar]
- Patients with higher body mass index treated with direct/novel oral anticoagulants (DOAC/NOAC) for atrial fibrillation experience worse clinical outcomes. Int J Cardiol. 2020;301:90-95. doi: 10.1016/j.ijcard.2019.10.035
- [CrossRef] [PubMed] [Google Scholar]
- Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
- [CrossRef] [PubMed] [Google Scholar]
- Management of venous thromboembolism in morbid obesity with rivaroxaban or warfarin. Ann Pharmacother. 2022;56:1315-1324. doi: 10.1177/10600280221089008
- [CrossRef] [PubMed] [Google Scholar]
- Real-world effectiveness and safety of rivaroxaban versus warfarin among non-valvular atrial fibrillation patients with obesity in a US population. Curr Med Res Opin. 2021;37:881-890. doi: 10.1080/03007995.2021.1901223
- [CrossRef] [PubMed] [Google Scholar]
- Multi-center retrospective study evaluating the efficacy and safety of apixaban versus warfarin for treatment of venous thromboembolism in patients with severe obesity. Pharmacotherapy. 2022;42:119-133. doi: 10.1002/phar.2655
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and extremes in body weight. Circulation. 2019;139:2292-2300. doi: 10.1161/CIRCULATIONAHA.118.037955
- [CrossRef] [PubMed] [Google Scholar]
- Safety and efficacy of direct oral anticoagulants in comparison with warfarin across different BMI ranges: A systematic review and meta-analysis. Ann Med Surg (Lond). 2022;77:103610. doi: 10.1016/j.amsu.2022.103610
- [CrossRef] [Google Scholar]
- Direct oral anticoagulants in the treatment of acute venous thromboembolism in patients with obesity: A systematic review with meta-analysis. Pharmacol Res. 2021;163:105317. doi: 10.1016/j.phrs.2020.105317
- [CrossRef] [PubMed] [Google Scholar]
- Effectiveness of direct oral anticoagulants in obese adults with atrial fibrillation: A systematic review of systematic reviews and meta-analysis. Front Cardiovasc Med. 2021;8:732828. doi: 10.3389/fcvm.2021.732828
- [CrossRef] [PubMed] [Google Scholar]
- Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: Updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
- [CrossRef] [PubMed] [Google Scholar]
- Prescribing pattern of oral anticoagulants in patients with obesity. J Pharm Pract. 2022;35:248-255. doi: 10.1177/0897190020969276
- [CrossRef] [PubMed] [Google Scholar]
- Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. doi: 10.1016/j.jbi.2008.08.010
- [CrossRef] [PubMed] [Google Scholar]
- The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208
- [CrossRef] [PubMed] [Google Scholar]
- Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: Communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119-2126. doi: 10.1111/jth.13140
- [CrossRef] [PubMed] [Google Scholar]
- Apixaban: Lexi-drugs. Riverwoods, IL: Wolters Kluwer Health, Inc; Available from: http://online.lexi.com [Last accessed on 2020 Oct 19]
- [Google Scholar]
- Rivaroxaban: Lexi-drugs. Riverwoods, IL: Wolters Kluwer Health, Inc; Available from: http://online.lexi.com [Last accessed on 2020 Oct 19]
- [Google Scholar]
- Direct oral factor Xa inhibitors in patients with morbid obesity-Author's reply. Lancet Haematol. 2019;6:e447. doi: 10.1016/S2352-3026(19)30171-1
- [CrossRef] [PubMed] [Google Scholar]
- Anticoagulant therapies and outcomes in obese patients with acute venous thromboembolism. Thromb Res. 2020;187:56-62. doi: 10.1016/j.thromres.2020.01.011
- [CrossRef] [PubMed] [Google Scholar]
- Direct-acting oral anticoagulant choice for stroke prevention in obese patients with atrial fibrillation. Can J Cardiol. 2021;37:1489-1492. doi: 10.1016/j.cjca.2021.04.004
- [CrossRef] [PubMed] [Google Scholar]
- Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI =50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: 10.1002/phar.2651
- [CrossRef] [PubMed] [Google Scholar]
- Comparing safety and efficacy of direct oral anticoagulants versus warfarin in extreme obesity. J Pharm Pract. 2023;36:1375-1382. doi: 10.1177/08971900221116809
- [CrossRef] [PubMed] [Google Scholar]
- Direct oral anticoagulants in obese patients with venous thromboembolism: Results of an expert consensus panel. Am J Med. 2023;136:523-533. doi: 10.1016/j.amjmed.2023.01.010
- [CrossRef] [PubMed] [Google Scholar]
- Successful use of rivaroxaban achieving therapeutic anti-factor Xa levels in a morbidly obese patient with acute intermediate-high risk pulmonary embolism. J Vasc Bras. 2023;22:e20230056. doi: 10.1590/1677-5449.202300562
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic drug monitoring of direct oral anticoagulants in patients with extremely low and high body weight-pilot study. J Clin Med. 2023;12:4969. doi: 10.3390/jcm12154969
- [CrossRef] [PubMed] [Google Scholar]
SUPPLEMENTAL TABLES
| Major bleeding |
|
| CRNMB |
|
ISTH: International Society on Thrombosis and Hemostasis, CRNMB: Clinically relevant non-major bleeding
| Recommended dose |
| Standard dose |
| VTE: • Apixaban 10 mg twice daily for 7 days followed by 5 mg twice daily • Rivaroxaban 15 mg twice daily with food for 21 days followed by 20 mg once daily with food |
| AF: • Apixaban 5 mg twice daily • Rivaroxaban 20 mg once daily with food |
| Hemodialysis: • Use of apixaban in patients receiving hemodialysis. |
| Dose adjusted |
| AF: • Apixaban – SCr >2.5 mg/dL or age >80 years: 2.5 mg twice daily • Rivaroxaban – CrCl 15–50 mL/min: 15mg once daily |
| Non-recommended dose |
| AF or VTE: • Use of inappropriate dose based on indication, dosage adjustment, or lack of dosage adjustment |
| VTE only: • Use of inappropriate lead in phase (i.e., inappropriate dose or duration based on individual agent) |
| Hemodialysis: • Use of rivaroxaban in patients receiving hemodialysis |
VTE: Venous thromboembolism, AF: Atrial fibrillation, SCr: Serum creatinine, CrCl: Creatinine clearance
| Outcome | DOAC (n=15) | Warfarin (n=17) | P-value |
|---|---|---|---|
| Treatment failure, n(%) | 0 | 1 (6) | 0.611 |
| VTE Recurrence | 0 | 1 (6) | - |
| Stroke, TIA, systemic embolism | 0 | 0 | - |
| Major bleeding or CRNMB, n(%) | 2 (13) | 4 (24) | 0.596 |
| Drop in hemoglobin ≥2 g/dL | 1 (7) | 2 (12) | 0.497 |
| Transfusion of ≥2 units of RBC or whole blood | 1 (7) | 2 (12) | 0.497 |
| Symptomatic bleeding in critical area or organ | 0 | 0 | - |
| Bleeding leading to death | 0 | 0 | - |
| CRNMB | 1 (7) | 2 (12) | 1.00 |
VTE: Venous thromboembolism, TIA: Transient ischemic attack, BMI: Body mass index, CRNMB: Clinically relevant non-major bleeding, RBC: Red blood cells, DOAC: Direct oral anticoagulants







