Translate this page into:
Assessing the knowledge, attitude, and practice of hospital-based pharmacists in reporting adverse drug reactions in Lagos, Nigeria
*Corresponding author: Foluke Adenike Ayeni, PhD, Department of Clinical Pharmacy and Biopharmacy, University of Lagos, Akoka, Lagos, Nigeria. faayeni@unilag.edu.ng
-
Received: ,
Accepted: ,
How to cite this article: Ayeni FA, Olugbake OA, Ambeke YP. Assessing the knowledge, attitude, and practice of hospital-based pharmacists in reporting adverse drug reactions in Lagos, Nigeria. Am J Pharmacother Pharm Sci. 2024:12.
Abstract
Objectives:
Adverse drug reactions (ADRs) are one of the major causes of morbidity and mortality associated with medication use in patients. Prompt reporting of all ADRs is the best way to address this issue. The objectives of this study are to assess the knowledge, attitude, and practice (KAP) of hospital pharmacists toward ADR reporting in selected public and private hospitals in two local government areas (LGAs) of Lagos State.
Materials and Methods:
A cross-sectional study was conducted among hospital pharmacists in Ikeja and Surulere LGAs using pretested and validated, self-administered questionnaires. Associations between demographic variables and KAP levels were evaluated using descriptive analysis and a Chi-squared test. The level of significance was set at P < 0.05.
Results:
A total of 100 questionnaires were received and analyzed; with 40% males and 60% females, majority of who were under 30 years. Pharmacists in public hospitals demonstrated a higher knowledge and attitude scores to ADR reporting at 90% and 81.7% respectively, while higher practice scores were seen in private hospitals at 37.5%. Overall, good knowledge and attitude scores of 89% and 82%, respectively, were reported, but poor practice scores of 23% were obtained across both hospital sectors. There was a degree of association between higher education levels and a positive attitude to ADR reporting at 3.37 (95% confidence interval: 0.99–11.49, P = 0.049).
Conclusion:
Hospital pharmacists in Lagos State have a high level of knowledge and positive attitude to ADR reporting but there is low practice, especially in the public sector.
Keywords
Adverse drug reaction
Knowledge
Attitude
Practice
Hospital pharmacist
Lagos state
INTRODUCTION
Adverse drug reactions (ADRs) are defined as any noxious, undesired, or unintended response to a therapeutic agent, which may be expected or unexpected, and may occur at dosages used for the prophylaxis, diagnosis, or treatment of disease, or for modifying physiological function. They do not include therapeutic failures, poisoning, accidental, or intentional overdoses.[1] Also defined as a “noxious and unwanted response to a medicine” by the European Medicine Agency,[2] ADRs are a broad term used to describe unwanted and dangerous effects caused by the use of medications. A classification of ADRs as suggested by Thomson and Rawlins in 1981, groups ADRs mainly into Type A and Type B ADR reactions. However, this has been modified to include types C, D, E, and F.[1] Type A reactions are unusual responses to medications administered at therapeutic doses. They are much more common and less fatal than Type B reactions which are unrelated to the dose and pharmacological action of the medication. Type C reactions occur due to an accumulated dose of a long-term medication; Type D reactions are delayed reactions, which occur long after the medication has been used; Type E reactions occur on withdrawal of a medication; and Type F reactions occur when a medication therapy is ineffective and considered as a failed therapy. ADRs differ from side effects, which are unintended effects, within an expected range of therapy, when a medication is used at normal doses.[3] ADRs are one of the major causes of morbidity and mortality associated with drug use. ADRs can cause or prolong hospital admissions and are increasingly becoming a growing public health concern with 15% of all patients affected by ADRs and 0.32% of all inpatients experiencing fatal ADRs.[4] ADRs are also among the top 10 causes of increasing healthcare cost in the United States and Europe.[5]
The primary tool for reporting ADRs in Nigeria is a structured in-take form known as the “Adverse Drug Reactions Form” (ADR Form). This form is similar to the United Kingdom’s yellow form with a fully completed ADR form, known as the Individual Case Safety Report. Nigeria is in a major crisis of under reporting ADRs.[5,6] With only 16,500 out of 80,000 ADR forms submitted back to the National Agency for Food and Drug Administration (NAFDAC) after being distributed nationwide for 12 years (2004–2016), this falls short of a World Health Organization’s criterium of 200 reports per million inhabitants per year.[5,6]
It is estimated that with a growing population of about 213 million people in 2021, about 42,600 reports should be submitted annually.[7] However, studies have revealed underreporting of ADRs in Nigeria. Opadeyi et al.[8] revealed poor pharmacovigilance documentation in tertiary hospitals of the South-South zone of Nigeria with the maximum number of ADR reports as 26 in a single year. Another study by Ohaju-Obodo and Iribhogbe[9] showed low reporting rates among resident doctors based in Lagos and Edo states, with 25% of all ADRs observed reported. This finding leaves more than a third of all observed ADR cases unreported. Oreagba et al.[10] discovered low reporting rates among community pharmacists in Lagos state with only 3% reporting ADRs to the National Pharmacovigilance Center (NPC). In light of these findings, there seems to be a paucity of data on ADR reporting among pharmacists practicing in hospitals. How knowledgeable are hospital pharmacists about ADRs and ADR reporting in Lagos state, Nigeria? What has been the general attitude and practice of hospital pharmacists toward ADR reporting in Lagos, Nigeria? These are the answers this research seeks to find. These data would produce new insights into a growing problem with a focus on hospital pharmacists in the Southwestern region of Nigeria, which had not been previously studied.
MATERIALS AND METHODS
Study setting
This cross-sectional study was carried out in Lagos State, located in the southwestern part of Nigeria. There are five administrative divisions which are Ikeja, Badagry, Ikorodu, Lagos, and Epe, and these divisions are divided into 20 Local Governments Areas (LGAs) and 37 Local Council Development Areas.[11] For the purpose of this research, two LGAs under the Ikeja division were chosen, namely, Ikeja and Surulere. These LGAs were selected due to the high number of public and private hospitals situated within the regions. Twenty-eight sites were visited. In Ikeja, 1 public tertiary hospital and 13 private hospitals were visited, and in Surulere, two public tertiary hospitals, one public general hospital, and 11 private hospitals were visited.
Study population
The study population was hospital pharmacists practicing in the two selected LGAs. All full-time hospital pharmacists within the study population, who voluntarily consented to participate, were included in the study. All intern and National Youth Service Corps pharmacists in hospitals and other members of the healthcare team such as doctors, dentists, nurses, medical laboratory scientists, physiotherapists, radiographers, dieticians, medical records officers, and health attendants were excluded from the study.
Study procedure
The questionnaire employed in this study was adapted from similar studies conducted in Nigeria[12] and the United Kingdom,[13,14] with inferences from a systematic review by Khalil and Huang.[15] The questionnaire was made up of four sections: Section A, which collected participant’s demographic data were categorical variables, reported as numbers and percentages; Sections B, C, and D included a Likert scale type questionnaire to assess knowledge, attitude, and practice (KAP) of ADRs and ADR reporting. There were 15 questions graded on a 2-point Likert scale (0–1), assessing respondents’ knowledge of ADRs, with one point awarded for each correct response, and 0 points for a wrong response. A maximum of 15 points was obtained and later converted to a percentage. There were 12 questions that assessed respondents’ attitude toward ADR reporting. These questions were graded on a 5-point Likert scale (0–4). A maximum of 48 points was obtainable and later converted to a percentage.
There were equally 15 questions graded on a 2-point Likert scale as well, which assessed respondents’ practice toward ADR reporting. One point was awarded for each correct response and 0 points for each wrong response. A maximum of 15 points was obtainable and was later converted to percentages. The questionnaires were administered through Google Forms and distributed through WhatsApp and Emails to hospital pharmacists in selected hospitals within Ikeja and Surulere LGAs. Hospitals were selected based on capacity, number of patients seen, and number of full-time pharmacists employed.
Sample size
The sample size was calculated using Slovin’s formula as shown:
n = N/(1+Ne2)
Where n is the sample size, N is the population number, and e is the margin of error.
At 90% confidence interval, e = 0.1
n = 1421/[1 + (1421)(0.12)]
n = 93.4
The sample size was 93.
Using an attrition rate of 10% based on a standard for survey data,
n = 93 + 9.32 = 102.3 ≈ 102
A convenient sampling of 100 hospital pharmacists working in Ikeja and Surulere LGAs was performed during the course of the study.
Data analysis
Sample data collected from questionnaires distributed through Google Forms were converted into a Microsoft Excel spreadsheet for ease of data management and analyzed using STATA 13 software (College Station, USA [StataCorp, 2013]). Before the analysis, all negatively worded items in the knowledge questions were reversed scored. Bivariate associations between demographic variables and KAP levels were evaluated using the Chi-squared test or Fisher’s exact tests, as relevant. Binomial logistic regression was conducted to predict KAP factors associated with ADR reporting. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed for each predictor variable. The level of significance was set at < 0.05.
Ethical approval
Ethical approval was obtained from the Health Research and Ethics Committee, Lagos University Teaching Hospital (HREC, LUTH), Idi-Araba in accordance with ethical conventions, with assigned number ADM/DSCST/HREC/APP/4944. Informed consent was obtained from each respondent, and response anonymity as well as confidentiality was maintained.
RESULTS
Demographics of respondents
A total of 100 questionnaires were received and analyzed. Of the participants, 40% were males, and 66% were under the age of 30. The majority 78% were awarded a Bachelor of Pharmacy degree, and 75% had <5 years of working experience [Table 1]. There was no statistically significant difference between sociodemographic characteristics and place of practice of pharmacists (P > 0.05).
| Variables | Private hospital n (%) | Public hospital n (%) | Total n (%) | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 15 (37.5) | 25 (41.7) | 40 (40.0) | 0.677 |
| Female | 25 (62.5) | 35 (58.3) | 60 (60.0) | |
| Age (in years) | ||||
| 21–30 | 28 (70.0) | 38 (63.3) | 66 (66.0) | 0.421 |
| 31–40 | 11 (27.5) | 15 (25) | 26 (26.0) | |
| 41–50 | 1 (2.5) | 6 (10) | 7 (7.0) | |
| 51–60 | 0 (0.0) | 1 (1.7) | 1 (1.0) | |
| Marital status | ||||
| Single | 29 (72.5) | 34 (56.7) | 63 (63.0) | 0.108 |
| Married | 11 (27.5) | 26 (43.3) | 37 (37.0) | |
| Level of education | ||||
| First (B.Pharm) | 33 (82.5) | 78 (78.0) | 45 (75.0) | 0.454 |
| First (Pharm.D) | 2 (5.0) | 2 (3.3) | 4 (4.0) | |
| Masters | 5 (12.5) | 10 (16.7) | 15 (15.0) | |
| WAPCP* | 0 (0.0) | 3 (5.0) | 3 (3.0) | |
| Number of years in practice | ||||
| 1–5 years | 31 (77.5) | 44 (73.3) | 75 (75.0) | 0.099 |
| 6–10 years | 9 (22.5) | 7 (11.6) | 16 (16.0) | |
| 11–15 years | 0 (0.0) | 4 (6.7) | 4 (4.0) | |
| 16–20 years | 0 (0.0) | 4 (6.7) | 4 (4.0) | |
| >20 years | 0 (0.0) | 1 (1.7) | 1 (4.0) | |
Pharmacist’s knowledge of ADR
Assessment of pharmacists’ general knowledge of ADR reporting showed that 95% knew the correct definition of ADRs and 69% knew the correct type of ADRs [Table 2].
| Knowledge of ADR reporting | Response | Private hospital n (%) | Public hospital n (%) | Total n (%) | P-value |
|---|---|---|---|---|---|
| 1. ADRs are side effects commonly experienced by a patient using a drug. | Yes | 15 (37.5) | 21 (35.0) | 36 (36.0) | 0.799 |
| 2. ADRs are unexpected effects of a drug when it is being used at normal doses | Yes | 38 (95.0) | 57 (95.0) | 95 (95.0) | 1.000 |
| 3. ADRs are restricted to orthodox medicines only. | Yes | 1 (2.5) | 0 (0.0) | 1 (1.0) | 0.218 |
| 4. ADRs are caused by POMs only | Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| 5. ADRs can be caused by error, drug abuse or misuse. | Yes | 34 (85.0) | 47 (78.3) | 81 (81.0) | 0.253 |
| 6. All ADRs are known during clinical trials. | Yes | 0 (0.0) | 3 (3.0) | 3 (3.0) | 0.249 |
| 7. All ADRs are dose dependent. | Yes | 3 (7.5) | 10 (16.7) | 13 (13.0) | 0.314 |
| 8. Only life-threatening reactions to a drug should be reported. | Yes | 2 (5.0) | 2 (3.3) | 4 (4.0) | 0.677 |
| 9. Reporting and documentation of all ADRs is important. | Yes | 40 (100.0) | 60 (100.0) | 100 (100.0) | - |
| 10. ADRs are a major cause of hospital mortality and morbidity. | Yes | 31 (77.5) | 45 (75.0) | 76 (76.0) | 0.932 |
| 11. Would you be able to report an ADR if it were to occur in your health-care facility? | Yes | 34 (85.0) | 53 (88.3) | 87 (87.0) | 0.607 |
| 12. Are you aware of the ADR reporting channel in Lagos state/Nigeria? | Yes | 23 (57.5) | 38 (63.3) | 61 (61.0) | 0.811 |
| 13. Which of the following are types of ADR’s | Type A, B, C, D, E, F and G | 27 (67.5) | 42 (70.0) | 69 (69.0) | 0.404 |
| Type 1, 2, 3, 4, 5, 6 and 7 | 1 (2.5) | 1 (1.7) | 2 (2.0) | ||
| Known, unknown and common, uncommon | 2 (5.0) | 7 (11.7) | 9 (9.0) | ||
| Reversible and irreversible | 3 (7.5) | 6 (10.0) | 9 (9.0) | ||
| Do not know | 7 (17.5) | 4 (6.7) | 11 (11.0) |
ADR: Adverse drug reaction, POMs: Prescription only medicines
All the respondents, 100% agreed that reporting and documentation of all ADRs were important and 87% were confident in their ability to report ADRs. In addition, 61% were aware of the correct ADR reporting channels while 75% knew the appropriate regulatory agency to obtain ADR forms from, of which 62% and 38% were public and private hospital pharmacists, respectively. Overall, general knowledge scores were 89%.
Information on the sources of information and regulatory bodies used for ADRs forms are shown in Figures 1 and 2, respectively. There was also no statistically significant difference between knowledge of pharmacists and place of practice (P > 0.05).
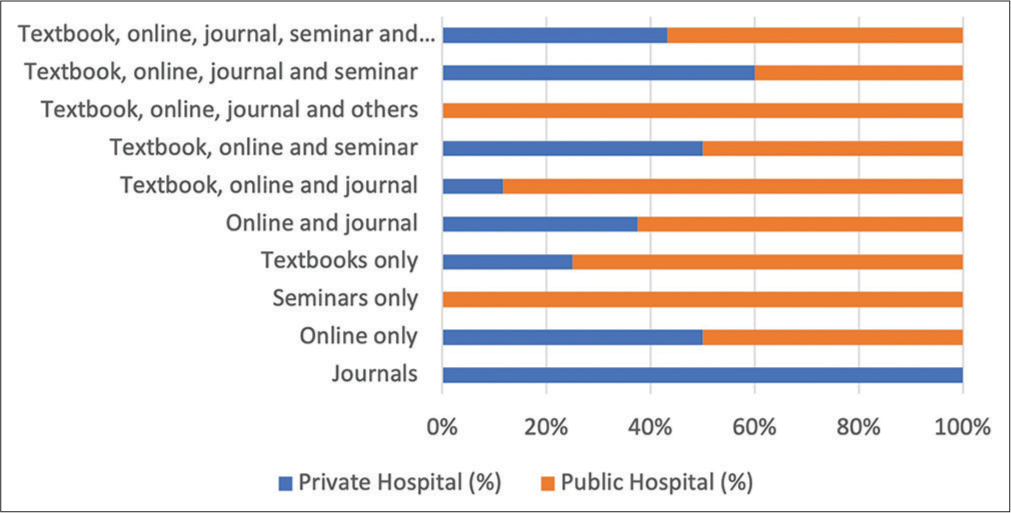
- Sources of information on adverse drug reactions.
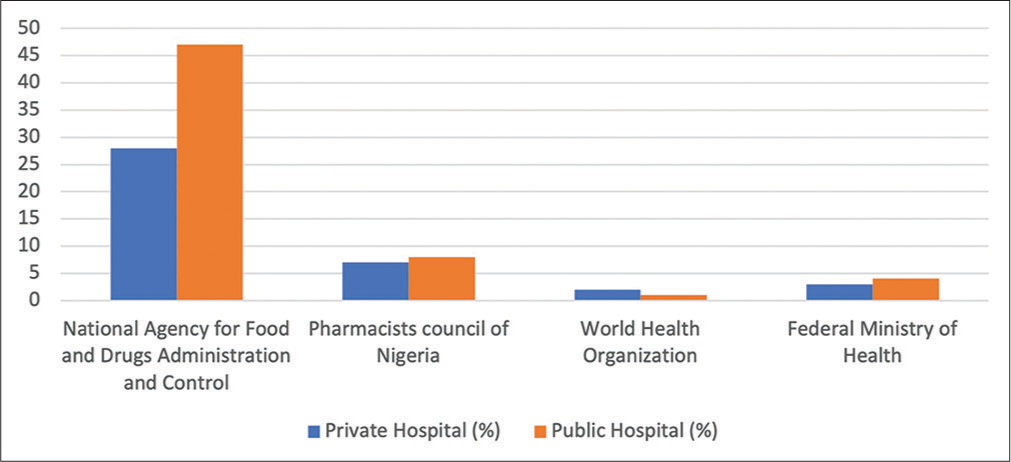
- Regulatory body to obtain adverse drug reaction forms.
Pharmacist’s attitude toward ADR reporting
Over half of the respondents, 59% were willing to report ADRs (64.4% were from public hospitals and 35.6% from private hospitals) [Table 3a]. Furthermore, 75% agreed that it was the pharmacist’s responsibility to report ADRs and 69% saw the necessity for training on post-marketing surveillance to identify and report ADRs. While there were mixed feelings regarding payment of incentives and what type of ADRs ought to be reported, 68% of the respondents believed that reporting ADRs increases the value of pharmacists in the healthcare sector. Similar proportions of pharmacists in both, private and public hospital settings, strongly agreed that training on post-marketing surveillance is vital in identifying and reporting ADRs (67.5 versus 70.0%, respectively) and that patient’s safety and wellbeing takes the highest priority in ADR reporting (75.0 versus 76.7%, respectively) [Table 3b]. However, a higher proportion of pharmacists in private hospital settings, 42.5%, disagreed to being more likely to report severe or lethal ADRs than mild and easily resolved ones, compared to pharmacists in public settings, 26.7%. A statistically significant difference was observed in pharmacist’s beliefs that ADR reporting should be made mandatory by law to address under-reporting [Table 3c].
| Attitude of pharmacists toward ADR reporting | Response | Private hospital n (%) |
Public hospital n (%) | Total n (%) | P-value |
|---|---|---|---|---|---|
| 1. I am willing and interested in reporting ADRs I come across in my practice. | SA | 21 (52.5) | 38 (63.3) | 59 (59.0) | 0.555 |
| A | 18 (45.0) | 21 (35.0) | 39 (39.0) | ||
| U | 1 (2.5) | 1 (1.7) | 2 (2.0) | ||
| D | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 2. It is my professional responsibility as a pharmacist to report ADRs. | SA | 26 (65.0) | 49 (81.7) | 75 (75.0) | 0.059 |
| A | 14 (35.0) | 11 (18.3) | 25 (25.0) | ||
| U | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| D | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 3. Reporting ADRs increases the value of pharmacists in the healthcare sector. | SA | 25 (62.5) | 43 (71.7) | 68 (68.0) | 0.598 |
| A | 13 (32.5) | 14 (23.3) | 27 (27.0) | ||
| U | 2 (5.0) | 2 (3.3) | 4 (4.0) | ||
| D | 0 (0.0) | 1 (1.7) | 1 (1.0) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 4. Training on post-marketing surveillance is vital in identifying and reporting ADRs. | SA | 27 (67.5) | 42 (70.0) | 69 (69.0) | 0.661 |
| A | 13 (32.5) | 17 (28.3) | 30 (30.0) | ||
| U | 0 (0.0) | 1 (1.7) | 1 (1.0) | ||
| D | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 5. Patient confidentiality is important in ADR reporting. | SA | 28 (70.0) | 46 (76.7) | 74 (74.0) | 0.411 |
| A | 11 (27.5) | 10 (16.7) | 21 (21.0) | ||
| U | 1 (2.5) | 2 (3.3) | 3 (3.0) | ||
| D | 0 (0.0) | 2 (3.3) | 2 (2.0) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
ADR: Adverse drug reaction, SA: Strongly agree, A: Agree, U: Undecided, D: Disagree, SD: Strongly disagree
| Attitude of pharmacists toward ADR hospital reporting |
Response | Private n (%) | Public hospital n (%) | Total n (%) | P-value |
|---|---|---|---|---|---|
| 4. Training on post-marketing surveillance is vital in identifying and reporting ADRs. | SA | 27 (67.5) | 42 (70.0) | 69 (69.0) | 0.661 |
| A | 13 (32.5) | 17 (28.3) | 30 (30.0) | ||
| U | 0 (0.0) | 1 (1.7) | 1 (1.0) | ||
| D | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| SA | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 5. Patient confidentiality is important in ADR reporting. | SA | 28 (70.0) | 46 (76.7) | 74 (74.0) | 0.411 |
| A | 11 (27.5) | 10 (16.7) | 21 (21.0) | ||
| U | 1 (2.5) | 2 (3.3) | 3 (3.0) | ||
| D | 0 (0.0) | 2 (3.3) | 2 (2.0) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 6. The patient’s safety and well-being takes the highest priority in ADR reporting. | SA | 30 (75.0) | 46 (76.7) | 76 (76.0) | 0.951 |
| A | 9 (22.5) | 13 (21.7) | 22 (22.0) | ||
| U | 1 (2.5) | 1 (1.7) | 2 (2.0) | ||
| D | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 7. I would not report previously unknown ADRs that had not been documented. | SA | 2 (5.0) | 1 (1.7) | 3 (3.0) | 0.351 |
| A | 7 (17.5) | 6 (10.0) | 13 (13.0) | ||
| U | 8 (20.0) | 7 (11.7) | 15 (15.0) | ||
| D | 15 (37.5) | 28 (46.7) | 43 (43.0) | ||
| SD | 8 (20.0) | 18 (30.0) | 26 (26.0) | ||
| 8. I would be more likely to report severe/lethal ADRs than mild and easily resolved ADRs. | SA | 3 (7.5) | 9 (15.0) | 12 (12.0) | 0.393 |
| A | 14 (35.0) | 21 (35.0) | 35 (35.0) | ||
| U | 3 (7.5) | 5 (8.3) | 8 (8.0) | ||
| D | 17 (42.5) | 16 (26.7) | 33 (33.0) | ||
| SD | 3 (7.5) | 9 (15.0) | 12 (12.0) |
ADR: Adverse drug reaction, SA: Strongly agree, A: Agree, U: Undecided, D: Disagree, SD: Strongly disagree
| Attitude of pharmacists toward ADR reporting | Response | Private hospital n (%) | Public hospital n (%) | Total n (%) | P-value |
|---|---|---|---|---|---|
| 9. I would be more motivated to report ADRs if incentives are paid. | SA | 10 (25.0) | 15 (25.0) | 25 (25.0) | 0.856 |
| A | 9 (22.5) | 17 (28.3) | 26 (26.0) | ||
| U | 7 (17.5) | 7 (11.7) | 14 (14.0) | ||
| D | 10 (25.0) | 17 (28.3) | 27 (27.0) | ||
| SD | 4 (10.0) | 4 (6.7) | 8 (8.0) | ||
| 10. I am confident in my ability to report ADRs. | SA | 12 (30.0) | 30 (50.0) | 42 (42) | 0.155 |
| A | 21 (52.5) | 24 (40.0) | 45 (45.0) | ||
| U | 4 (10.0) | 5 (8.3) | 9 (9.0) | ||
| D | 3 (7.5) | 1 (1.7) | 4 (4.0) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 11. ADR reporting should be made mandatory by law to address under-reporting. | SA | 7 (17.5) | 24 (40.0) | 31 (31.0) | 0.032 |
| A | 22 (55.0) | 25 (41.7) | 47 (47.0) | ||
| U | 8 (20.0) | 4 (6.7) | 12 (12.0) | ||
| D | 3 (7.5) | 7 (11.7) | 10 (10) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 12. Every hospital should have an ADR committee to A enforce ADR reporting. | SA | 27 (67.5) | 38 (63.3) | 65 (65.0) | 0.792 |
| A | 11 (27.5) | 19 (31.7) | 30 (30) | ||
| U | 2 (5.0) | 2 (3.3) | 4 (4.0) | ||
| D | 0 (0.0) | 1 (1.7) | 1 (1.0) | ||
| SD | 0 (0.0) | 0 (0.0) | 0 (0.0) |
ADR: Adverse drug reaction, SA: Strongly agree, A: Agree, U: Undecided, D: Disagree, SD: Strongly disagree
Pharmacist’s practice toward ADR reporting
Most pharmacists (87%) had experienced complaints of ADRs from patients [Table 4], out of which 36.8% and 63.2% were from private and public hospitals, respectively. However, only 21% of respondents had reported an ADR in the past three months. During that time, only one person had reported more than 10 ADRs. In addition, 35% were part of continuous courses or refresher studies on ADRs after graduation. The majority of pharmacists had a positive attitude towards ADR reporting with an attitude score of 82%. The overall practice score was 23% [Table 5].
| Practice of pharmacists toward ADR reporting | Response | Private hospital n (%) | Public hospital n (%) | Total n (%) | P-value |
|---|---|---|---|---|---|
| 1. Have you ever experienced a situation in which a patient complained of an adverse reaction to a drug? | Yes | 32 (80.0) | 55 (91.7) | 87 (87.0) | 0.089 |
| 2. ADRs are commonly detected by: | Patient | 18 (45.0) | 20 (33.3) | 38 (38.0) | |
| Pharmacist | 18 (45.0) | 27 (45.0) | 45 (45.0) | ||
| Physician | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Others | 4 (10.0) | 13 (21.7) | 17 (17.0) | ||
| 3. Have you reported an ADR in the past 3 months? | Yes | 10 (25.0) | 11 (18.3) | 21 (21.0) | 0.322 |
| 4. Number of ADRs reported in past 3 months? | 1 | 5 (12.5) | 5 (8.3) | 10 (10) | 0.561 |
| 2–5 | 4 (10.0) | 7 (11.7) | 11 (11.0) | ||
| 6–10 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| >10 | 1 (2.5) | 0 (0.0) | 1 (1.0) | ||
| None | 30 (75.0) | 48 (80.0) | 78 (78.0) | ||
| 5. How do you report ADRs? (Please select none if no ADR was reported) | Physically drop hard copy | 9 (22.5) | 12 (20.0) | 21 (21.0) | 0.489 |
| Send hard copies | 10 (25.0) | 10 (16.7) | 20 (20.0) | ||
| On-line reporting | 5 (12.5) | 5 (8.3) | 10 (10.0) | ||
| None | 16 (40.0) | 33 (55.0) | 49 (49.0) | ||
| 6. Nature of ADR (s) reported? (Please select none if no ADR was reported) | Mild | 9 (22.5) | 4 (6.7) | 13 (13.0) | 0.063 |
| Moderate | 8 (20.0) | 10 (16.7) | 18 (18.0) | ||
| Severe | 0 (0.0) | 3 (5.0) | 3 (3.0) | ||
| All of the above | 3 (7.5) | 2 (3.3) | 5 (5.0) | ||
| None | 20 (32.8) | 41 (67.2) | 61 (61.0) | ||
| 7. Have you had any continuous education, training or refresher study on ADR reporting? | Yes | 12 (30.0) | 23 (38.3) | 35 (35.0) | 0.392 |
| 8. Source of ADR (s) reported (choose all applicable options) | POM | 21 (52.5) | 25 (41.7) | 46 (46) | 0.208 |
| OTC drug | 7 (17.5) | 6 (10.0) | 13 (13.0) | ||
| Herbal medicine | 9 (22.5) | 22 (36.7) | 31 (31.0) | ||
| None reported | 3 (7.5) | 7 (11.67) | 10 (10.0) | ||
| 9. What measures do you take in case of an ADR? | Withdraw causative drug | 36 (90.0) | 50 (83.3) | 86 (86.0) | 0.721 |
| Treat symptoms with another drug | 2 (5.0) | 3 (5.0) | 5 (5.0) | ||
| Nothing, reaction resolves on its own | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Provide counseling to patients | 1 (2.5) | 4 (6.7) | 5 (5.0) | ||
| Others | 1 (2.5) | 3 (5.0) | 4 (4.0) |
ADR: Adverse drug reaction, POMs: Prescription only medicines, OTC: Over the counter
| Private hospital | Public hospital | Total | ||||
|---|---|---|---|---|---|---|
| Pass (%) | Fail (%) | Pass (%) | Fail (%) | Pass (%) | Fail (%) | |
| Knowledge score | 35 (87.5) | 5 (12.5) | 54 (90.0) | 6 (10.0) | 89 (89.0) | 11 (11.0) |
| Attitude score | 33 (82.5) | 7 (17.5) | 49 (81.7) | 11 (18.3) | 82 (82.0) | 18 (18.0) |
| Practice score | 15 (37.5) | 25 (62.5) | 8 (13.3) | 52 (86.7) | 23 (23.0) | 77 (77.0) |
ADR: Adverse drug reaction
Predictors of pharmacist KAP toward ADR reporting
The demographics of pharmacists were used as predictors of ADR KAP at P < 0.05. These results revealed a degree of association between a higher level of education and attitude to ADR reporting with a crude OR of 3.37 (95% CI: 0.99–11.49), even though the CI crossed ONE. Otherwise, there was no significant degree of the association at 95% CI [Table 6].
| Variable | Regression variable | COR | SE | P-value | CI |
|---|---|---|---|---|---|
| Knowledge | Sex | 1.31 | 0.89 | 0.695 | 0.34–4.96 |
| Age | 1.45 | 1.11 | 0.628 | 0.32–6.54 | |
| Marital status | 1.27 | 0.95 | 0.745 | 0.29–5.49 | |
| Highest level of education | 1.22 | 0.60 | 0.691 | 0.46–3.21 | |
| Number of years in practice | 1.19 | 0.49 | 0.676 | 0.53–2.67 | |
| Attitude | Sex | 0.93 | 0.55 | 0.904 | 0.29–2.94 |
| Age | 1.23 | 0.68 | 0.712 | 0.41–3.65 | |
| Marital status | 1.67 | 1.05 | 0.412 | 0.49–5.73 | |
| Highest level of education | 3.37 | 2.11 | 0.049 | 0.99–11.49 | |
| Number of years in practice | 0.67 | 0.20 | 0.188 | 0.38–1.21 | |
| Practice | Sex | 0.83 | 0.42 | 0.719 | 0.30–2.25 |
| Age | 1.11 | 0.52 | 0.821 | 0.44–2.79 | |
| Marital status | 0.75 | 0.43 | 0.613 | 0.24–2.30 | |
| Highest level of education | 0.95 | 0.29 | 0.879 | 0.53–1.73 | |
| Number of years in practice | 1.15 | 0.28 | 0.555 | 0.72–1.86 |
ADR: Adverse drug reaction, COR: Crude odd ratio, SE: Standard error, CI: Confidence interval, CI - 0.99-11.49: CI crossed 1, though barely significant.
DISCUSSION
The theoretical knowledge of ADRs is necessary as it provides an understanding as to why reporting and documentation of all ADRs is vital in reducing morbidity, mortality, and financial burden on patients. Overall, results from this study revealed that 95% of pharmacists across private and public hospitals were knowledgeable about the definition of ADRs (private to public hospital ratio of 0.97). This finding is similar to a Chinese study,[16] which reported that 89% of hospital pharmacists were knowledgeable on ADR definitions, and another in Egypt, at 94%.[17] Given the rigorous training in pharmacy school, pharmacists should be well-versed on definitions and classifications of ADRs. Theoretically, pharmacists should be able to differentiate between a side effect and an ADR, which would aid identification and reporting. However, there seemed to be a decline in knowledge with regard to the types of ADR when compared to the definition, as 69% of respondents correctly chose the Rawlins classification of ADRs, which are type A, B, C, D, E, and F.[1] This decline from 95% to 69% could have been as a result of a lack of training or refresher courses postgraduation or few encounters of ADR presentations in patients. We also found positive knowledge on ADR reporting as 100% of all pharmacists agreed that all ADRs should be reported and documented, regardless of severity, and 87% were confident in their knowledge on how to use the ADR form. These results are higher than what was observed in a study conducted in Jordan which revealed that 73% of pharmacists saw the importance of reporting all ADRs.[18] It was observed that 70% of hospital pharmacists in Japan understood the ADR reporting system.[19] This was similar to what our study found with 61% of respondents knowing the correct ADR reporting channel in Lagos State/Nigeria, which is either through the NAFDAC state offices, the zonal pharmacovigilance centers or directly to the NPC in Abuja.[5] Overall, the knowledge score was high at 89%, higher than reports from an Ethiopian study (57.1%).[20] This discrepancy could have been because the latter study involved all healthcare professionals, as opposed to this study which focused only on hospital pharmacists, who are custodians of medications. There was no significant degree of association between the demographics of pharmacists and the prediction of knowledge about ADRs.
In this study, there was an overall positive attitude score of 82% which indicates a greater predisposition to reporting ADRs as soon as it is encountered, and this mirrored the overall attitude scores in Ethiopia (78.9%).[20] The majority of pharmacists in this study were willing to report ADRs encountered at their hospitals as they saw it as part of their responsibility in providing pharmaceutical care to patients, and strongly agreed that doing this increases the value of pharmacists in the healthcare sector. This finding is consistent with studies in China; where 85% of respondents believed that ADR reporting was the responsibility of pharmacists,[16] and in Lebanon[21] and Saudi Arabia,[22] where 79% and 91% of pharmacists, respectively, saw it as a professional obligation.
Given that all erroneously filled or incomplete ADR forms are not sent to the International Drug Monitoring Center in Uppsala,[5] the willingness to be trained on the identification and filling of ADR forms is vital as this aids the submission of accurate reports. This goes hand-in-hand with the patient being the main focus in ADR reports and is a positive step in ensuring patient safety and medication efficacy. The willingness to learn is seen in this study where almost a third of all pharmacists, more in public hospitals than private hospitals, strongly agree on trainings and the patients being the main focus while reporting. Similar results were seen by Alshabi et al., where 87% of hospital pharmacists in Saudi Arabia agreed to training,[22] and another in Jordan, where 84% believed that ADR reporting improved quality care of patients.[18] However, there were fewer agreements on making ADR reporting mandatory by law in this study as 31% of respondents strongly agreed to mandatory reporting. This is lower compared to reports in Saudi Arabia, where 69% of pharmacists felt the need for mandatory reporting.[23]
In this study, there seems to be a degree of association between a higher level of education and positive attitude to reporting ADRs, at p value = 0.049.
The result obtained states a point estimate of 3.37 with a 95% confidence interval of 0.99 to 11.49. While the p-value indicates that there might be a statistically significant effect, the confidence interval just barely includes the value of 1. This proximity to 1 in the confidence interval suggests that we cannot be confident that there is a true effect, and the association might not be truly statistically significant. This might have occurred because the confidence interval is wide, suggesting a large degree of uncertainty in the estimate. It might also be due to the small sample size in this study, which leads to less precise estimates. Despite the CI including 1, the lower bound is very close to 1, indicating that almost all of the confidence interval suggests an association. Pharmacists with a higher education level beyond their first degrees were 3 times more likely to have a positive attitude to reporting ADRs than those with B. Pharm and Pharm.D degrees as first degrees only. This attitude could most likely be due to exposure, as graduate professionals, to an in-depth curriculum on specific areas of interests/specialization as opposed to generalized areas in undergraduate studies, which would facilitate the practice of what was learned.
There were low practice scores of 23% across both hospitals as opposed to 62.8% recorded in Jordan.[18] The majority of pharmacists in this study (87%) had encountered ADRs, consistent with studies in Australia (88.4%)[24] and Saudi Arabia (86.1%)[22] but only 21% had reported an ADR in the past three months. This results to a ratio of identifying ADRs to reporting them at 0.24 which differs from the higher ADR reporting to identification ratio of 0.83 in China,[16] 0.90 in Malaysia,[25] and 0.94 in Saudi Arabia.[22] Furthermore, there were close similarities between those who had refresher courses and/or training (35%), and reporting rates (21%) which emphasizes the fact that the majority of pharmacists in this study were first-degree holders as consistent with the Chinese study, which revealed that lower education levels were associated with lower reporting rates.[16] Nevertheless, this difference could have occurred due to variations in the duration of reported ADRs as this study inquired about reporting rates within three months, while the referenced studies asked within a duration of six months.
Comparing pharmacists from both sectors reported higher knowledge and attitude scores with public hospital pharmacists than private, but higher practice scores with private hospital pharmacists than public. Consistent results were found in a study by Hu et al., which could be due to the fact that pharmacists in the public sector have had more years of experience and would have had more encounters with ADRs, making them more knowledgeable and better predisposed to reporting such.[16] However, an increased work burden, lack of time, and a smaller pharmacist-to-patient ratio, due to migration from the country in recent years, may have contributed to poor practice. Furthermore, a better work environment, electronic medical records, and less work burden may have given privately owned hospitals an edge over public hospitals. In addition to this, the lower number of private hospital pharmacists in this study may have given the pharmacists in public hospitals a greater advantage with knowledge and attitude scores.
Limitation
The small sample size of this study might have served as a limitation. Further studies with a larger sample size are recommended.
CONCLUSION
Hospital pharmacists in Lagos have a high level of knowledge and positive attitude to ADR reporting but there is low practice, especially in the public sector. The introduction of an ADR reporting app to aid spontaneous reporting by patients, caregivers, pharmacists, and other healthcare personnel might improve reporting rates.
Acknowledgments
The authors would like to thank all public and private hospital pharmacists who were part of the study.
Ethical approval
Ethical approval was obtained from the Health Research and Ethics Committee, Lagos University Teaching Hospital (HREC, LUTH), Idi-Araba in accordance with ethical conventions, with assigned number ADM/DSCST/HREC/APP/4944.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
None.
References
- Adverse drug reactions in older adults: A narrative review of the literature. Eur Geriatr Med. 2021;12:463-73. doi: 10.1007/s41999-021-00481-9
- [CrossRef] [PubMed] [Google Scholar]
- Adverse drug reaction. Netherlands: European Medicines Agency; Available from: https://www.ema.europa.eu/en/glossary/adverse-drug-reaction#:~:text=a%20noxious%20and%20unintended%20response%20to%20a%20medicine [Last accessed on 2023 Aug 28]
- [Google Scholar]
- Adverse drug reactions - clinical pharmacology In: MSD Manual Professional Edition. 2021. Available from: https://www.msdmanuals.com/professional/clinical-pharmacology/adverse-drug-reactions/adverse-drug-reactions#v1109644 [Last accessed on 2023 Aug 28]
- [Google Scholar]
- Incidence and economic burden of adverse drug reactions in hospitalization: A prospective study in Korea. J Korean Med Sci. 2023;38:e56. doi: 10.3346/jkms.2023.38.e56
- [CrossRef] [PubMed] [Google Scholar]
- Addressing the under-reporting of adverse drug reactions in public health programs controlling HIV/AIDS, Tuberculosis and Malaria: A prospective cohort study. PLoS One. 2018;13:e0200810. doi: 10.1371/journal.pone.0200810
- [CrossRef] [PubMed] [Google Scholar]
- The importance of pharmacovigilance: Safety monitoring of medicinal products Geneva: World Health Organization; 2002. p. :42.
- [Google Scholar]
- Nigeria: Population 1950-2020. 2021. Statista. Available from: https://www.statista.com/statistics/1122838/population-of-nigeria [Last accessed on 2023 Dec 26]
- [Google Scholar]
- Assessment of the state of pharmacovigilance in the South-South zone of Nigeria using WHO pharmacovigilance indicators. BMC Pharmacol Toxicol. 2018;19:27. doi: 10.1186/s40360-018-0217-2
- [CrossRef] [PubMed] [Google Scholar]
- Extent of pharmacovigilance among resident doctors in Edo and Lagos states of Nigeria. Pharmacoepidemiol Drug Saf. 2010;19:191-195. doi: 10.1002/pds.1724
- [CrossRef] [PubMed] [Google Scholar]
- The knowledge, perceptions and practice of pharmacovigilance amongst community pharmacists in Lagos state, south west Nigeria. Pharmacoepidemiol Drug Saf. 2010;20:30-35. doi: 10.1002/pds.2021
- [CrossRef] [PubMed] [Google Scholar]
- About Lagos. 2022. Lagos State Government. Available from: https://lagosstate.gov.ng/about-lagos [Last accessed on 2023 Dec 26]
- [Google Scholar]
- Awareness, knowledge, attitude and practice of adverse drug reaction reporting among health workers and patients in selected primary healthcare centres in Ibadan, southwestern Nigeria. BMC Health Serv Res. 2019;19:926. doi: 10.1186/s12913-019-4775-9
- [CrossRef] [PubMed] [Google Scholar]
- Attitudes and knowledge of hospital pharmacists to adverse drug reaction reporting. Br J Clin Pharmacol. 2008;51:81-86. doi: 10.1046/j.1365-2125.2001.01306.x
- [CrossRef] [PubMed] [Google Scholar]
- Barriers to reporting of adverse drugs reactions: A cross sectional study among community pharmacists in United Kingdom. Pharm Pract (Granada). 2017;15:931. doi: 10.18549/pharmpract.2017.03.931
- [CrossRef] [PubMed] [Google Scholar]
- Adverse drug reactions in primary care: A scoping review. BMC Health Serv Res. 2020;20:5. doi: 10.1186/s12913-019-4651-7
- [CrossRef] [PubMed] [Google Scholar]
- Knowledge, attitude and practice of hospital pharmacists in central China towards adverse drug reaction reporting: A multicenter cross-sectional study. Front Pharmacol. 2022;13:823944. doi: 10.3389/fphar.2022.823944
- [CrossRef] [PubMed] [Google Scholar]
- Spontaneous adverse drug reaction reporting by community pharmacists: Preparedness and barriers. Saudi Pharm J. 2022;30:1052-1059. doi: 10.1016/j.jsps.2022.04.006
- [CrossRef] [PubMed] [Google Scholar]
- Knowledge, attitude and practices of pharmacovigilance and adverse drug reaction reporting among pharmacists working at Alkarak governorate, Jordan. Biomed Pharmacol J. 2022;15:967-978. doi: 10.13005/bpj/2432
- [CrossRef] [Google Scholar]
- Knowledge, attitudes, and practice of hospital pharmacists regarding pharmacovigilance and adverse drug reaction reporting in Japan. Hosp Pharm. 2021;56:7-16.
- [CrossRef] [Google Scholar]
- Barriers to reporting adverse drug reaction among health care providers in Ethiopia. Research Square [Preprint]; 2022 doi: 10.21203/rs.3.rs-2272439/v1
- [CrossRef] [Google Scholar]
- Medication safety knowledge, attitude, and practice among hospital pharmacists in Lebanon. J Eval Clin Pract. 2018;25:323-339. doi: 10.1111/jep.13082
- [CrossRef] [PubMed] [Google Scholar]
- Knowledge, attitude and practice of hospital pharmacists towards pharmacovigilance and adverse drug reaction reporting in Najran, Saudi Arabia. Saudi Pharm J. 2022;30:1018-1026. doi: 10.1016/j.jsps.2022.04.014
- [CrossRef] [PubMed] [Google Scholar]
- Medication safety knowledge, attitude, and practice among hospital pharmacists in tertiary care hospitals in Saudi Arabia: A multi-center study. Arch Public Health. 2021;79:130. doi: 10.1186/s13690-021-00616-1
- [CrossRef] [PubMed] [Google Scholar]
- Community pharmacists' knowledge and perspectives of reporting adverse drug reactions in Australia: A cross-sectional survey. Int J Clin Pharm. 2018;40:878-889. doi: 10.1007/s11096-018-0700-2
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacists' experiences on adverse drug reaction: 10 years later. Front Pharmacol. 2022;13:932942. doi: 10.3389/fphar.2022.932942
- [CrossRef] [PubMed] [Google Scholar]






