Translate this page into:
The Calabar bean and physostigmine: from African ethno-jurisprudence to medicinal discovery and modern pharmacotherapeutics
*Corresponding author: Ashiwel S. Undieh, MPharm PhD Professor of Neuroscience and Pharmacology, Department of Biomedical Sciences, City University of New York School of Medicine, 160 Convert Avenue, New York, NY 10031, United States. aundieh@med.cuny.edu
-
Received: ,
Accepted: ,
How to cite this article: Obi JO, Ikeme AC, Nwakama PE, et al. The Calabar bean and physostigmine: from African ethno-jurisprudence to medicinal discovery and modern pharmacotherapeutics. Am J Pharmacother Pharm Sci 2023;1.
Abstract
Physostigmine, or eserine, is an alkaloid found in the Calabar bean (eséré), Physostigma venenosum (Balfour). The scientific discovery of physostigmine played a pivotal role in our understanding of chemical neurotransmission and the emergence of pharmacology as a science. The Efik people of Old Calabar, in present day Cross River State of Nigeria, used the bean as an ordeal poison to determine if persons accused of certain allegations such as witchcraft were guilty or innocent. Scottish missionaries working in Old Calabar witnessed eséré ordeal trials and attended to patients inadvertently poisoned by the plant. Perplexed by the ordeal trials and concerned for their patients, the missionaries sent reports of their observations and samples of the beans to scientists back in Britain for study. Evaluations of the plant material for its chemical constituents and biological activity led to the discovery of its active principle, physostigmine. Further explorations with physostigmine led to the discovery of acetylcholine and chemical neurotransmission, and the Nobel Prize for Physiology or Medicine in 1936. From a classic practice of ethno-jurisprudence, the world came to understand the chemical basis of synaptic transmission, and the nature of substrate-enzyme and ligand-receptor interactions as underlying principles in biochemistry and pharmacology. Numerous medicines including physostigmine have been developed based on these principles and are being used in current pharmacotherapy. The rich history of eséré plant and its physostigmine alkaloid anchors a compelling story of the role natural products have played in the discovery of modern therapeutic agents. Moreover, the story highlights the reality that probably many more medicinal plants in Africa remain to be explored for their chemical constituents as potential leads in breakthrough drug discovery.
Keywords
Calabar bean
Ethno-jurisprudence
Medicinal discovery
Ethnopathic medical practice
Physostigmine
Ethnopharmacology
INTRODUCTION
The discovery of physostigmine and its specific biological action is one of the most fascinating scientific advances of all time. This discovery is variously claimed as a pivotal point in the development of the scientific disciplines of pharmacology and biochemistry, as well as the modern professions of pharmacy and medicine. Interest in physostigmine has remained high, not only because of the multiple roles the alkaloid has played in disease treatment and parsing out complex pharmacological mechanisms, but also because of its captivating history in apparently valid ethnological practice of jurisprudence. Physostigmine, also known as eserine, is a natural alkaloid found in the Calabar bean, or eséré (Efik), Physostigma venenosum (Balfour).[1] The Calabar bean [Figure 1] and its alkaloid, physostigmine, underline an inseparable tale of the bean’s use as an ordeal poison and of systematic laboratory explorations that led to seminal advances and novel discoveries in chemistry, physiology, and pharmacology. In particular, the rich history of the Calabar bean is one among many that need to be continually recounted to increase awareness on the contributions of plants and Africa as the origin or original source of diverse modern drugs and dietary supplements used around the world.

- Illustration of the Calabar bean (Physostigma venenosum) twigs, flowers, pod, and seeds.
GEOGRAPHIC AND HISTORICAL SETTING
Extending from the Bakassi peninsula west across the Cross River estuary and north toward the Oban foothills of Southeastern Nigeria lies the area once referred to as Old Calabar. Long before Calabar’s famous but brief role as the Capital of Nigeria, the people of Old Calabar were a vibrant community ruled by Obongs and councils of elders and priests. The use of the Calabar bean, or eséré as the natives called it, in traditional jurisprudence existed before recorded history. The scientific discovery of the Calabar bean and its introduction to allopathic medical practice, however, commenced with the European exploration of West Africa in the 15th century [Table 1].
 |
As early as 1469, Fernão Gomes, a Portuguese merchant began trading at the Guinea coast of West Africa. By 1475, his ships had crossed the equator, and he consolidated at a base in the Gold Coast, present day Ghana. By 1493, the Portuguese had extended their ships to São Thomé and Fernando Po islands, from where they could carry out trade at the delta region of the Niger river. Portuguese merchants continued to monopolize trade in goods and slaves on West African shores until the end of the 16th century when other European countries including French, English and Dutch, also set up their own trading posts at the popular Slave Coast and the so-called Oil Rivers including Old Calabar.[2,3]
As one of the booming trading posts in the Oil Rivers area, the name Old Calabar was reported to have been coined by the Dutch in the 17th century, probably from confounding the inhabitants with the Kalabari people who had already moved westward from the region. The people who lived on the Cross River estuary by this time were the Efik people that are found in present day Cross River and Akwa Ibom states of Nigeria.
The Efik people had a prominent male group, known as the Ekpe or Leopard society, from which they appointed chiefs and elders to govern the affairs of their community. They had a duke or a king chosen as the head of the society, and the ruler of the community. The duke together with the chiefs, imposed laws for governing Efik society. Punishment for breaking the law or committing crimes ranged from a death penalty to fines and trade embargoes against neighboring towns.[4] The Efik people of Old Calabar, like many other African societies at the time, had a system of religious and mystical beliefs, including ancestor veneration (which was prescribed) and witchcraft (which was proscribed). Accusations of witchcraft activity were taken seriously and had grave consequences because those involved were usually subjected to the deadly eséré ordeal trial according to societal customs. The eséré ordeal was only one of many customs in Efik society that served to maintain law and order, community and communality. By the 18th and 19th centuries, however, eséré ordeal began to be suspiciously employed as a political weapon within the Ekpe society as it was sometimes thought to be misused to settle fights, take out threatening rivals, and bring down powerful individuals.[2]
TRIAL BY ORDEAL
In ancient times, trials by ordeal were the norm and were generally accepted to determine the guilt or innocence of accused individuals, and to settle disputes in many African societies. The believed involvement of all-knowing ancestral spirits gave the ordeal trials a particularly feared reputation. There were many types of ordeal carried out, including ordeal by fire, ordeal of hot water or oil, and ordeal by poison. Regardless of the ordeal method, one thing was common - the accused persons had to perform a test, or carry out an act, which would harm them or even cost them their lives unless they were innocent.[5] In no case has the scientific basis of the ordeal test been studied and vindicated as with the eséré test.
William Freeman Daniell was known to be the first outsider to witness a Calabar bean poison ordeal, during an ordeal ceremony of the Efik people. Dr. Daniell was a physician in the British Army and went on exploratory missions to Africa.
He was also a botanist and had a passion for collecting and describing plants native to the countries he visited.[2] Between 1840 and 1845, Daniell’s explorations led him to Old Calabar in West Africa where, with the king’s approval, he was able to witness a Calabar bean poison ordeal.[6,7] He documented his observations in a report which he gave to the Ethnological Society of Edinburgh in 1846. In his report, a court of justice made up of the king and his chiefs usually gathered for an ordeal ceremony. In this ceremony, accused individuals were brought before the court to judge the offense. If the evidence suggested guilt, the accused was coerced to go through the eséré ordeal, which they referred to in pidgin as “chopping nut.” The poison was prepared by crushing the seeds and steeping them in water until a milky white liquid was obtained. The accused was then given the potion to drink and was instructed to walk around for a time. If the accused happens to vomit within that time, he or she was considered innocent and was set free. Conversely, an accused person who died after ingesting the potion was considered guilty as charged.[8]
In a broader context, the Efiks, like many other West African peoples, used one form of divination or another in attempts to determine the cause of unexplained events. The eséré ordeal was a type of divination by which the supernatural powers of the gods and ancestors were invoked through the action of the bean to help uncover and destroy witchcraft.[4] Some may argue, however, that these beliefs about the Calabar bean poison ordeal, and its use in determining one’s innocence were based solely on superstition. Given the now-known toxicity of the potion, however, and the reality that not everyone who took the potion died, there has to have been some logical basis to the practice. For example, an accused who was certain of their innocence might have quickly swallowed the poison, which did not give enough time for its absorption from the buccal cavity; moreover, swallowing the potion quickly as a bolus was more likely to trigger the vomit reflex, resulting in expulsion of the poison before enough of it got absorbed to cause harm. On the other hand, a person who was guilty might have tended to slowly sip the potion out of intense fear; which allowed for absorption of the poison from the oral cavity, and failure to trigger vomiting, leading to their death.[9] Wood and LaWall have also suggested that the chief priest who directed the ordeal could influence the outcome of the exposure by choosing to use either fresh beans which better induced vomiting or using fully ripened beans and in the form of a concentrated liquid potion which was more poisonous.[10] But these are mere speculations put forward by outsiders who may think they ought to be able to decipher this otherwise enigmatic practice. Regardless of whether eséré could truly help determine if one was guilty or not, the narration from Daniell’s exploration sparked great interest in Europe.
TO EDINBURGH BOTANIC GARDENS
The Edinburgh Botanic Gardens started as far back as 1670 when the Edinburgh medical school was established. At that time, physicians and apothecaries had to extemporaneously compound their own medicines for treating ailments, and they did this mostly from plants. The need for access to plant sources inspired a group of physicians to start a herb garden, now known as the Royal Botanic Garden Edinburgh (RBGE). In 1676, the Materia Medica, which in today’s vocabulary will be the study of pharmacology and toxicology, was established as a subject taught to medical students to broaden their knowledge on medicines. Botany was also taught to students at the Edinburgh medical school, starting from 1738.[11] In 1834, John Hutton Balfour, a notable biologist and botanist began his medical profession in Edinburgh.[12] Balfour played an important role in creating the Botanical Society of Edinburgh in 1836, and the Edinburgh Botanical Club in 1838. He was appointed as the Regius Keeper of RBGE and the Queen’s Botanist for Scotland.[13]
Balfour’s connections and role as keeper of the garden introduced him to the Calabar bean. Hope Waddell, a Scottish missionary of Old Calabar brought the beans back from one of his missions to Edinburgh. The beans sprouted and grew at the botanic gardens, but they failed to flower, which impeded the ability of the plant to be fully described. The missionaries in Old Calabar found it difficult to obtain the whole plant, and this difficulty was attributed to the fact that most natives of Old Calabar were reluctant to be associated with the plant or “they could not recognize the plant unless it was together with the fruit from which the beans came.”[14] Nevertheless, some missionaries including Zerub Baillie who was Balfour’s former medical student, successfully obtained some eséré leaves and flowers and took them back to Scotland. This enabled Balfour to provide the first complete description of the plant in 1861.[15] The plant was named P. venenosum (Balfour), both parts of the name being derived from two prominent characteristics of the plant. The stigma of the flower has an extraordinary appendage, resembling a bladder, hence physo, the Greek word for bladder, was combined with “stigma” to form the Genus. The species name venenosum was derived from the Latin word “venenum” for poison.[1]
Hidden within this narrative is the less recognized but probably significant role played by a former slave. Archibald Hewan was born in 1832 to John and Ann Hewan, enslaved Africans in Hamden, Jamaica. Just before Archie was born, Ann’s father, who was now a retired plantation owner living in Scotland, had donated money to the Church of Scotland to build a church and school for free and enslaved children in Hamden. Hewan was thus able to attend church and gain an education. At the age of 19, he travelled to Britain under the auspices of the Missionary Society with the goal to study medicine. As part of his curriculum at the Edinburgh medical school, he studied Materia Medica under Professor John Balfour who was now heading RBGE. The two became friends and colleagues thereafter. On licensure as a physician, Hewan left for Old Calabar where he established a thriving medical practice and was “reputed for his skills as a doctor and preacher”[16] Reverend Hope Waddell, Dr. Zerub Baillie, and Dr. Archibald Hewan were contemporaries and collaborators while in Old Calabar, and their lives had previously intersected in notable ways. The older Waddell was missionary to Jamaica and contributor to the Hamden church and school at the time Hewan was born; both Baillie and Hewan studied under Dr. Balfour at RBGE, and all three were missionaries working within about a 10-mile radius. Further, Hewan and Waddell learned to speak the Efik language well enough to communicate and to gain the trust and admiration of the people they served. Two factors, though, set Hewan apart. First, being Black probably helped to gain the trust of the natives for himself and his colleagues (although being the only black among dozens of sponsored missionaries sometimes meant he had to fight for justice for himself - as when he successfully sued his superiors for discriminatory treatment).[16] Secondly, Hewan was the foremost practicing botanist among them. He is reported to have been readily eager to undertake excursions to the jungles or up mountains with natives or European explorers. In one instance, when he left too hurriedly to pack specimen collection supplies, he ended up turning his book “choke full” of pressed plant parts of previously undocumented species. He regularly sent back specimens to Dr. Balfour at RBGE for classification and herbarium archiving. In one of his letters from 1862 that has been preserved, he wrote about sending a box containing seeds of the Calabar bean and pressed herbarium specimens to Dr. Balfour. It may not be too much of a stretch, therefore, to argue that the plant specimens and seeds the various donors, including Baillie and Waddell, were said to have given to Balfour and other scientists probably originated from Hewan. If so, then Hewan must have made substantial contributions to the evolution of the physostigmine science and story.
DISCOVERING THE ACTIVE PRINCIPLES AND CONFIRMING THE BIOLOGICAL ACTIVITY OF ESÉRÉ
Sir Robert Christison was known to have conducted the first toxicological research on P. venenosum.[17] He was one toxicologist that did not shy away from performing experiments on himself. He got the Calabar bean from Hope Waddell and experimented on himself by ingesting a part of the bean on an empty stomach. He was nearly poisoned, but luckily, he had soapy water from shaving which he used to self-induce emesis and survived. This experiment demonstrated that the substance was capable of producing potent and potentially fatal biological effects outside of the traditional Efik environment and usage. He wrote an account on his research observations which he published in 1855.[6] By the way, Christison was also known for studying the effects of cocaine by experimenting on himself at the age of 78.[11]
Christison’s experimentation with the Calabar bean paved the way for more thorough studies to be carried out on the plant. Thomas Richard Fraser, his assistant, advanced the studies after taking over from Christison as professor of Materia Medica and Therapeutics in 1877.[11] Fraser isolated the active principle of the Calabar bean in 1863, showing it to be an amorphous alkaloid present in the kernel of the bean.[7,18] Fraser named the alkaloid eserine based on the eséré name that the Efiks called the plant and bean. Jobst and Hesse, working independently, isolated a purified alkaloid from the seeds of the Calabar bean in 1864 and named it physostigmine.[19] In 1865, the pure crystalline form of the alkaloid was also obtained by Vee who decided to also name it serine.[20,21] Both names, eserine and physostigmine, have persisted and can be used interchangeably to describe the principal alkaloid present in the Calabar bean.
Physostigmine was determined to be the main alkaloid in the Calabar bean which caused its toxicity.[22] However, some other active principles were later identified to contribute to the biological effect. Some of these alkaloids include calabarine which was identified in 1876, but was later reported to be a mixture of degradation products.[23,24] Eseramine was identified in 1893,[24] physovenine in 1911,[22] and geneserine in 1915.[25,26] At least six alkaloids were isolated from the Calabar bean through 1989, including the aforementioned as well as eseridine,[27,28] isophysostigmine[29] and eseroline which is a degradation product of physostigmine.[30]
EXAMINING THE PHYSIOLOGICAL ACTIONS OF ESÉRÉ
The earliest studies on the physiological actions of physostigmine were conducted by Thomas Fraser. His studies demonstrated that the toxic effects of physostigmine could be antagonized or reversed by atropine, an alkaloid extracted from the deadly nightshade, Atropa belladonna.[31] He was able to explain the physiological action of physostigmine on the central nervous system, heart, pupil, voluntary muscles, and the intestines. His work provided great insight on drug antagonism, as he found that physostigmine antagonized the effects of atropine on the heart rate and the mydriatic effect of atropine on the pupil.[32,33] Conversely, atropine blocked many of the effects of physostigmine. The first reported medical utilization of physostigmine was in ophthalmic practice as a miotic. Atropine caused dilation of the pupil of the eye, for which ladies were wont to use extracts of the deadly nightshade as a cosmetic aid, sometimes with toxic side effects. At the time when the mydriatic effect of atropine was discovered, there was no known way of counteracting the pupillary dilation. Douglas Argyll Robertson, an ophthalmologist and friend of Thomas Fraser was popular for publishing several papers on eye diseases.[34,35] On learning about physostigmine from his friend Fraser, he used the drug in his practice as a pupil constrictor, and thus an antidote for the treatment of atropine-induced or poisoning-related mydriasis.[36]
Apart from its miotic effect on the pupil of the eye, physostigmine was found to relax the ciliary muscles and enhance the outflow of aqueous humor which reduced elevated intraocular pressure. Ludwig Laqueur, another ophthalmologist, identified this effect of physostigmine, and was the first to use the drug in the treatment of glaucoma. Laqueur suffered from glaucoma and decided to treat himself with physostigmine which worked temporarily to improve his eye function until he ultimately had surgery. Notwithstanding its time-limited and non-curative efficacy, physostigmine gained popularity afterwards in the treatment of glaucoma.[37,38]
Physostigmine was used or attempted for use in various other medical conditions. It was used for stimulation of the smooth muscle of the intestinal tract and urinary bladder in patients who underwent abdominal surgery and had difficulty with urinating or bowel movement.[39] In 1934, Mary Broadfoot Walker made the first attempt at using physostigmine for the treatment of myasthenia gravis, a neuromuscular disorder characterized by autoimmune inactivation of acetylcholine receptors supplying the voluntary muscles. Physostigmine was found to have no effect on the condition. Subsequently, Walker successfully treated a myasthenia gravis patient with neostigmine, a derivative of physostigmine with a longer duration of action.[40,41] Physostigmine was found to be useful in the treatment of Friedreich’s ataxia and other inherited ataxias.[42] More recently, an attempt was made to use physostigmine for the treatment of Alzheimer’s disease, but efficacy was limited by its short duration of action.[43]
A NOBEL PRIZE FOR THE SCIENCE OF ESÉRÉ ORDEAL POISONING
In the bid by scientists to elucidate its mechanism of action, physostigmine came to play what is arguably the most crucial role in the development of pharmacology as a scientific discipline born from a molecular understanding of chemical neurotransmission and drug-receptor interactions. Before studies on the mechanism of action of physostigmine, pharmacologists and physiologists generally believed that impulses were transmitted between nerves through an electric current. But adjacent neurons were known not to touch each other, leaving an intervening gap known as a synapse. The vexing question of how information flowed between nerves across the synapse was later addressed when it was found that the electrical signal carried by a nerve toward a synapse was transformed into the release of a hormone, called acetylcholine, which then diffused across the synapse to pass on the signal to the adjacent nerve cell.[44] This discovery only emerged as the mechanism of action of physostigmine was being investigated.[45]
The elucidation of the mechanism of action of physostigmine was a collective effort of multiple pharmacologists and biochemists over several decades of time and work. By 1906, Anderson observed that the constriction caused by physostigmine on the pupil persisted even when the nerves supplying the pupil were cut. The constriction was lost only when the cut nerves were allowed to degenerate. The accepted reason at the time for this disparity was that the newly cut nerves still had a chemical substance present that facilitated the effect of physostigmine, although no one knew what that chemical substance or transmitter was.[46] By 1911, Loewi and Mansfield observed that peripheral organs were more sensitive to electric stimulation in the presence of physostigmine.[47] In 1912, Loewi performed experiments on vagus nerve stimulation in rabbits with physostigmine and pilocarpine, an alkaloid that was known to exert parasympathomimetic effects. Loewi observed that vagus stimulation was increased in the presence of physostigmine, but not of pilocarpine.[48] By 1914, Dale established the muscarine-like and nicotine-like effects of the autonomic nervous system. In the course of these studies he noticed that the effects of acetylcholine in the body were short-lived and proposed that an esterase enzyme may be responsible for this brevity in action by rapidly degrading acetylcholine.[49] Fuhner, among others, then obtained data indicating that the mechanism of action of physostigmine was to inhibit the hydrolysis of acetylcholine by the tissues.[50]
Between 1921 and 1926, Loewi conducted arduous and extensive studies on humoral neurotransmission.[51] His conclusions demonstrated for the 1st time that chemical transmission between nerves, and from nerve to end organs, was possible.[52] He discovered that a chemical substance secreted at the neuromuscular junction was responsible for the electrical stimulation of the vagus nerve. He named this chemical substance vagusstoff and tried to isolate it for more studies.[53] His attempts at isolating vagusstoff failed because the substance was being degraded rapidly by an enzyme that was later identified as acetylcholinesterase (AChE). Loewi, however, finally succeeded in isolating this substance when he discovered that its enzymatic degradation could be prevented by physostigmine. He isolated vagusstoff, now known as acetylcholine, in 1926.[54] In 1936, Otto Loewi and Henry Dale were awarded the Nobel Prize in Physiology or Medicine for their discovery of acetylcholine and elucidation of chemical neurotransmission – an achievement that was primarily motivated by the quest to uncover the mechanism of action of physostigmine.
PHYSOSTIGMINE SYNTHESIS AND NEW ANALOGS
Just like the elucidation of the mechanism of action of physostigmine took many years, the elucidation of the structure of physostigmine was also an arduous and time-consuming task undertaken by multiple laboratories. Back in those days, the instrumental capabilities of spectroscopy – ultraviolet-visible, infrared, or mass spectrometry – were not available for structure elucidation as they are today. Researchers were limited to using chemical degradation strategies for determining the structure of alkaloids or other complex compounds. In 1925, two chemists at the University of Edinburgh, Edgar Stedman and George Barger successfully determined the structure of physostigmine as a 5-hydroxyindole structure, with an attached pyrrolidine ring.[55] Multiple researchers then began attempts to synthesize the compound in their laboratories. Due to the complexity of its structure, the synthesis of physostigmine required intermediate steps before conversion to the final product. Professor Robert Robinson of England hit a stumbling block where he synthesized an intermediate compound, l-eséréthole, but could not make further progress toward physostigmine.
Fortunately, Dr. Percy Julian, an African American chemist and research fellow at DePauw University in the United States [Figure 2], succeeded in converting his l-eséréthole into the next intermediate compound, eseroline; conversion of the latter to physostigmine was straightforward, thus winning him the race to the total synthesis of physostigmine [Figure 3]. Structural characterization studies showed the synthesized compound to be completely identical to the natural alkaloid.[56] Dr. Julian’s achievement was particularly noteworthy because as a Black man he was excluded from attending high school but he graduated at the top of his chemistry class at DePauw University; he was prevented from advancing to candidacy for the PhD degree at Harvard University but he eventually obtained his PhD from the University of Austria in 1931. He was denied tenure and promotion at his alma mater DePauw University, although he is considered the foremost American chemist having pioneered the chemical synthesis of industrial leads and medicinal compounds ranging from physostigmine to steroids and birth control medications.

- Dr. Percy Julian in his chemistry laboratory (courtesy of sciencehistory.org, Philadelphia, PA).
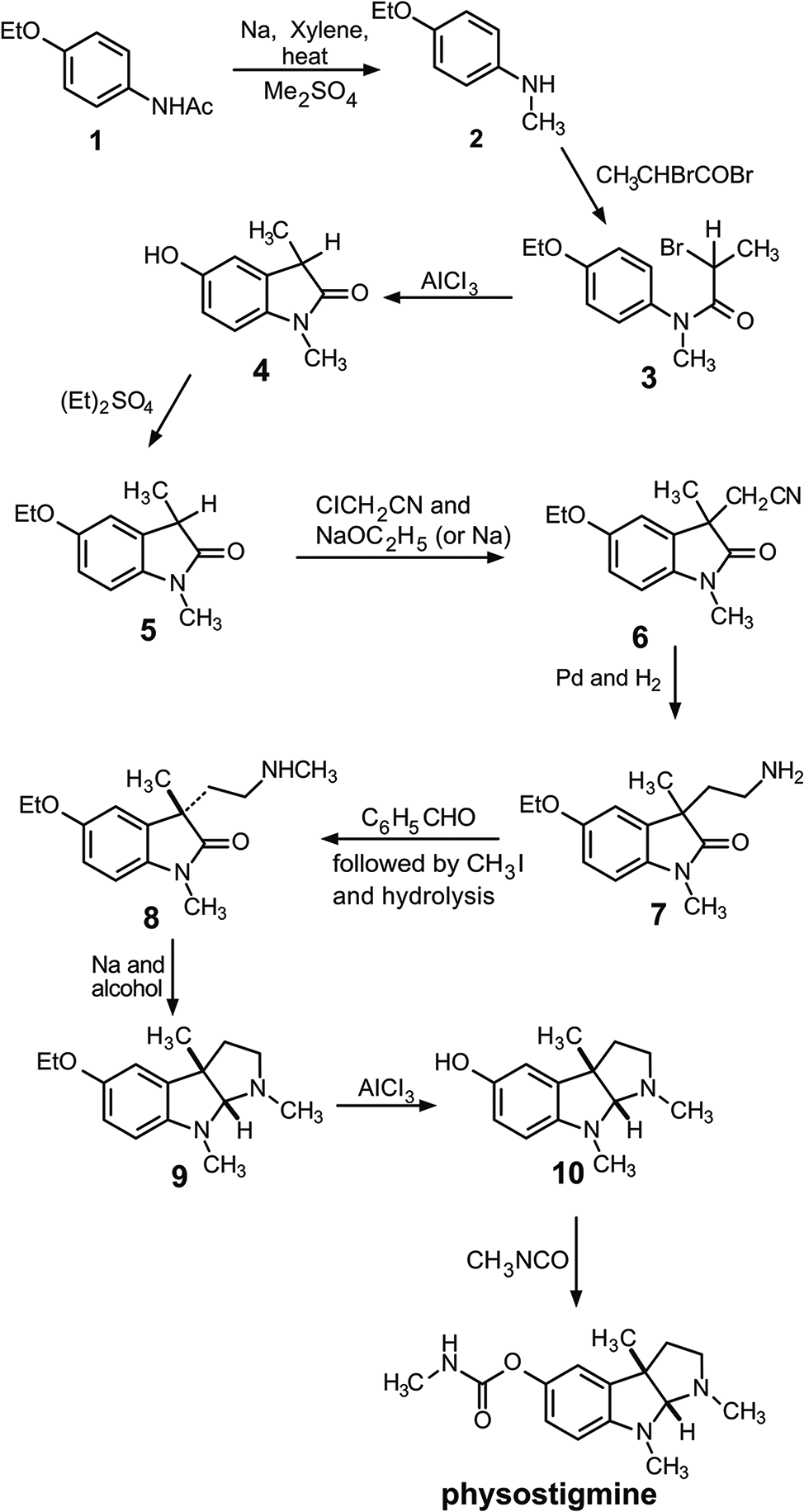
- First total synthesis of physostigmine (Julian and Pikl, 1935).
Other chemists focused on synthesizing physostigmine analogs with improved properties over the lead compound.[57-59]
As a drug, physostigmine has a relatively short duration of action, and some unwanted cholinergic side effects. This led chemists to attempt synthesizing derivatives of the compound to extend its duration of action and reduce its side effects. Miotine was synthesized by Stedman and Barger, but the compound did not perform pharmacologically as well as physostigmine. Other members of the physostigmine family in clinical use include neostigmine, pyridostigmine, demecarium, ambenonium, and rivastigmine [Table 2]. These compounds belong to the pharmacological class of reversible AChE inhibitors, and most share the structural feature of being carbamate derivatives. The carbamate moiety imparts pharmacological efficacy. Introduced by the Roche firm, neostigmine is a quaternary ammonium derivative of physostigmine that is longer acting and is clinically used in the treatment of myasthenia gravis and for gastrointestinal tract stimulation. Pyridostigmine, a close analog of neostigmine, is currently listed in the United States Pharmacopeia for use in myasthenia gravis.[45] An interesting insight in the structural features is the observation that linking two quaternary ammonium moieties can increase anticholinesterase potency and duration of action as is the case with the miotic agent demecarium which consists of 2 neostigmine molecules connected by a series of 10 methylene groups.[60] Ambenonium is another double quaternary compound and is used to treat myasthenia gravis.[60] Rivastigmine is highly lipophilic, ensuring its access into the brain via the blood-brain barrier, and as such the drug is currently marketed as oral capsules, solution, and transdermal patches for treatment of Alzheimer disease.[61]
 |
DRUGS OUT OF AFRICA
The history of pharmacology cannot be told without reference to the role that ethnopathy – traditional healing practices involving the use of plants and other natural substances – played in helping pharmacologists and physicians discover many modern therapeutics. From atropine and quinine to paclitaxel and artemisinin, plant-based ethnotherapeutic practices from all over the world have seeded much of the progress and tools of modern pharmacotherapy.[62,63] African traditional medicine is the oldest, and arguably the most diverse, traditional health-care system in human history.[64] The practice is deeply rooted in the cultural diversity, religious background, transferable knowledge, attitudes and beliefs of ethnomedicine practitioners in various African communities. Regardless of this diversity, the traditional health-care system in Africa is holistic in nature, in that it aims to heal the body, the mind, as well as sociocultural structures and networks from which one draws a sense of self, values, purpose, and support. From time immemorial, restoring the well-being of a person or community through traditional medicine was done through spiritualism, divination, or herbalism, based on the knowledge or beliefs of that community.[65] At present, however, we can see that the traditional system, though seminal and potentious, lacks broad applicability due to its oft blending with spiritism or divination, and its usual confinement to benefit members of each therapy’s custodial family or community. The eséré/ physostigmine story teaches the value of the scientific method in validating and expanding the benefits of African healing resources. In contrast with divination which is always secretive, uncertain, and exclusivist, science searches for objective, reproducible, and generalized explanations for natural phenomena while also sanctioning systematic strategies to exploit such phenomena for human and environmental benefit. Thus, a transition of African healing arts toward an objective system of ethnopathic practice is essential for the realization of the full potential of the resource in this 21st century.
A complete cataloguing of scientifically developed or validated African medicinal plants or admixtures is beyond the focus of this review. Granted that not all health-related beliefs or practices can withstand the objective scrutiny of the scientific method, we can learn from the past to not throw out the baby with the bath water but to separate the chaff from the grain. Adopting that approach could easily reveal many more medicinal plants in Africa that could be tapped and developed into innovative therapeutics for broad use. Indeed, an increasing number of scientific publications has been pointing to the value of exploring the potential of African medicinal plants for the development of new therapeutics.[66-68] However, significant progress is being hampered due to lack of documentation on the medicinal properties of these plants. Most traditional medicine practitioners in Africa acquire their knowledge from older generations of practitioners through word of mouth, and formal documentation of claims, methods of preparation and use, and healing outcomes is typically lacking. Moreover, to implement a thorough documentation of promising medicinal plants from Africa for investigative drug discovery, it is important to consider the concerns of traditional healers: Once they give out the secrets of their trade, they lose a heirloom and personally gain nothing in return. A strategy that rewards original curators of therapeutic herbal knowledge will go a long way to allay these concerns.
African governments and citizens spend substantial budgets on drug importation, yet only a minor fraction of the healthcare needs of most African countries are being met for their citizens. A proffered solution might be to develop indigenous African medicinal plants for use across the region, especially given the propitious business climate being promoted by the African Continental Free Trade Area initiative. Such development can proceed in at least two pathways depending on the capabilities and priorities of a sponsor government or community. The first is the conventional strategy of isolating pure active principles, extensively researching their biological properties, progressing the molecules through a billion-dollar and decades-long preclinical and clinical drug development program, and acquiring regulatory approval for marketing the products in multiple jurisdictions. This strategy is unaffordable to most African countries. The alternative strategy is to identify credible plant-based therapies, document their traditional usage in juxtaposition with modern diagnostic categories, establish telltale scientific tests to validate the biological activity and safety of the plant preparations, and then appropriately formulate and manufacture standardized preparations for introduction into mainstream healthcare. This alternate approach, which does not necessarily sacrifice proven medicinal benefit or safety, can be budget-friendly, fast, and accessible to most communities across the continent. As revenue streams from the endeavor mature, effort may then be directed toward more systematic research and chemical optimization of the leading constituents. Indeed, a team of seven African scientists recently developed a formulation of standardized neem leaf extract and zinc gluconate as a dietary supplement, and it took less than a year to go from concept to a marketed product (ASU, personal communication).
CONCLUSION AND FUTURE PERSPECTIVES
Current knowledge of many basic mechanisms in physiology and biochemistry would have been hampered considerably had early interest and attention not focused on the deadly poison ordeal of the Efik people of Old Calabar. The scientific community still marvels at the astute and translational abilities of the native Efik people – to have observed the effects of the wild plant, conceived a strategy to employ it in their traditional jurisprudence, and maintain a level of integrity while using the technique (it is conceivable that the potion worked largely within reason, else if everybody who took it died then it would not have been an effective way to tell the innocent from the guilty).
Physostigmine was the first alkaloid proven to act through specific inhibition of an enzyme target and the knowledge of its mechanism of action facilitated the development of a successful bioassay for the neurohumoral transmitter acetylcholine. The alkaloid has contributed most to the understanding of neurohumoral chemical transmission and mapping of cholinergic nerves, in relation to the heart, ganglionic transmission, neuromuscular transmission, vasodilator fibers, and sweat gland innervation. It also contributed much to the understanding of the function of the blood brain barrier. Eserine has also been the subject of classical experiments with atropine and curare on pharmacological antagonism. It played an important role in the understanding of the enzyme kinetics of AChE and has contributed to the elucidation of the configuration of the active center of this enzyme. At present, physostigmine has been supplanted by synthetic compounds for use in ophthalmic conditions and neuromuscular deficits, but the alkaloid remains of choice as an antidote for poisoning with atropine and atropine-like compounds.
Part of the motivation for this review was to highlight the contributions that the African environment and cultural practices have made to modern medicine. The hope is that this would inspire individuals, institutions and governments in Africa to look back to this rich natural resource for help in addressing some of the healthcare needs of the continent. Allopathic physicians, pharmacists, herbalists, and scientists could work together to develop a mechanism for mainstreaming proven traditional therapies for general use. Then the story of eséré and eserine will move from being an emblem of nostalgia to becoming a blueprint for integrated development of allopathic, osteopathic, and ethnopathic medical practices into a holistic system of healthcare for Nigeria, Africa, and the world.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflict of interest
There are no conflict of interest.
Financial support and sponsorship
None.
References
- Episodes in the story of physostigmine. Mol Interv. 2010;10:4-10.
- [CrossRef] [PubMed] [Google Scholar]
- The ordeal bean of old Calabar: The pageant of Physostigma venenosum in medicine. In: Plants in the Development of Modern Medicine. Massachusetts, United States: Harvard University Press; 1972.
- [Google Scholar]
- An Introduction to the History of West Africa (3rd ed). Cambridge, United Kingdom: Cambridge University Press; 1962.
- [Google Scholar]
- On the properties of the ordeal bean of old Calabar, Western Africa. Edinb Med J. 1855;1:193-204.
- [Google Scholar]
- On the characters, actions, and therapeutic uses of the ordeal bean of Calabar (Physostigma venenosum, balfour). Section 1: History, employment as an ordeal, botanical characters, etc. Edinb Med J. 1863;9:36-56.
- [Google Scholar]
- On the natives of old Callebar, West Coast of Africa. Edin N Philos J. 1846;40:313-327.
- [Google Scholar]
- Drug Discovery: A History. Chichester, UK: John Wiley and Sons Ltd.; 2005. p. :96-97.
- [CrossRef] [Google Scholar]
- The Dispensatory of the United States of America (21st ed). Philadelphia, PA: J.B. Lippincott Company; 1926. p. :840.
- [Google Scholar]
- History of Scottish Medicine. Vol 02. (2nd ed). London, Wellcome Historical Medical Museum: Bailliere, Tindall and Co.; 1932.
- [Google Scholar]
- The early toxicology of physostigmine: A tale of beans, great men and egos. Toxicol Rev. 2006;25:99-138.
- [CrossRef] [PubMed] [Google Scholar]
- On the physiological actions of the ordeal bean of Calabar, and on its antagonism to tetanus and strychnia-poisoning. Edinb Med J. 1867;12:999-1024.
- [Google Scholar]
- Description of the plant which produces the ordeal bean of Calabar. Trans R Soc Edinb. 1861;22:305-314.
- [CrossRef] [Google Scholar]
- The Life and Achievements of Dr Archibald Hewan. 2022. Available from: https://stories.rbge.org.uk/archives/36668 [Last accessed on 2023 Feb 03]
- [Google Scholar]
- On the moth of the eséré, or ordeal-bean of old Calabar. Ann Nat Hist. 1864;13:389-393.
- [CrossRef] [Google Scholar]
- Recherches Chimiques et Physiologiques Sur la Feve du Calabar (These) Paris: Nosoli 72; 1865.
- [Google Scholar]
- De l'alcaloide de la feve du Calabar et experiences physiologiques avec ce meme alcaloide. C R Soc Biol 1864:160-172.
- [Google Scholar]
- Pharmakologische untersuchungen uber das physostigmin und Calabarin. Arch Exp Path Pharmacol. 1876;5:401-454.
- [CrossRef] [Google Scholar]
- Etude sur les alcaloides de la feve de Calabar (II) La Geneserine nouvel alcaloid de la feve. Bull Soc Chim Fr. 1915;17:244-256.
- [Google Scholar]
- Etude sur les alcaloides de la feve de Calabar (IV) Synthese partielle de l'eserine et de la geneserine. Bull Soc Chim Fr. 1916;19:27-37.
- [Google Scholar]
- Ein neues physostigminderivat und seine pharmacologische Bedeutung. Berl Thierarztl Wschr. 1888;41-49:57-68.
- [Google Scholar]
- Reversible inhibition of acetylcholinesterase by eseroline, an opioid agonist structurally related to physostigmine (eserine) and morphine. Biochem Pharmacol. 1982;31:1233-1238.
- [CrossRef] [PubMed] [Google Scholar]
- On atropia as a physiological antidote to the poisonous effects of physostigma. Practitioner. 1870;4:65-72.
- [Google Scholar]
- Lecture on the Antagonism between the actions of active substances. Br Med J. 1872;618:485-487.
- [CrossRef] [PubMed] [Google Scholar]
- On the Calabar bean as a new agent in ophthalmic medicine. Edin Med J. 1863;8:815-820.
- [CrossRef] [Google Scholar]
- Ueber atropin und physostigmin in ihre wirkung auf den intraocularen druck. Ein beitrag zur therapie des glaucoms. Arch Ophthal. 1877;23:149-176.
- [CrossRef] [Google Scholar]
- Goodman and Gilman's the Pharmacological Basis of Therapeutics (11th ed). New York: McGraw Hill; 2006. p. :212.
- [CrossRef] [Google Scholar]
- Mary Broadfoot Walker (1888-1974): A historic discovery in myasthenia gravis. Eur Neurol. 2005;53:51-53.
- [CrossRef] [PubMed] [Google Scholar]
- Dr. Mary Walker-a Pioneer in the Treatment of Myasthenia Gravis United States: Myasthenia Gravis Association News, Autumn; 2002.
- [Google Scholar]
- On the physiological action of certain choline derivative and new methods for detecting choline. Br Med J. 1906;2:1788-1789.
- [Google Scholar]
- Drug Discovery: The Evolution of Modern Medicines Chichester, UK: John Wiley and Sons; 1985. p. :115-118.
- [Google Scholar]
- The paralysis of involuntary muscle. Part III On the action of pilocarpine, physostigmine, and atropine upon the paralyzed iris. J Physiol. 1906;33:414-438.
- [CrossRef] [PubMed] [Google Scholar]
- Uber den wirkungsmodus des physostigmins (III Mitteilung) Arch Exp Path Pharm. 1911;62:180-185.
- [CrossRef] [Google Scholar]
- Untersuchungen zur physiologie und pharmakologie des herzvagus. III Mitteiling: Vaguserregbarkeit und vagusgifte. Arch. Pharmakol. 1912;70-71:351-368.
- [CrossRef] [Google Scholar]
- The action of certain esters and ethers of choline and their relation to muscarine. J Pharmacol. 1914;6:147-190.
- [Google Scholar]
- Uber humorale uebertragbarkeit der herznervenwirkung (I. Mitteilung) Pflugers Arch Ges Physiol. 1921;189:239-242.
- [CrossRef] [Google Scholar]
- Uber humorale uebertragbarkeit der herznervenwirkung. (II. Mitteilung) Pflugers Arch Ges Physiol. 1921;193:201-213.
- [CrossRef] [Google Scholar]
- Uber humorale uebertragbarkeit der herznervenwirkung (XI. Mitteilung Uber den mechanismus der vaguswirkung von physostigmin und ergotamin) Pflugers Arch Ges Physiol. 1926;214:689-696.
- [CrossRef] [Google Scholar]
- Studies in the indole series. V. The complete synthesis of physostigmine (Eserine) J Am Chem Soc. 1935;57:755-757.
- [CrossRef] [Google Scholar]
- Acetylcholinesterase inhibitors from plants and fungi. Nat Prod Rep. 2006;23:181-199.
- [CrossRef] [PubMed] [Google Scholar]
- Pharmacological action of some analogues of physostigmine. J Pharmacol Exp Ther. 1931;43:413-444.
- [Google Scholar]
- Anticholinesterase agents In: Brunton LL, HilalDandan R, Knollmann BC, eds. Goodman and Gilman's the Pharmacological Basis of Therapeutics. New York: McGraw Hill; 2018. p. :173.
- [Google Scholar]
- Clinical pharmacology-the first 75 years and a view of the future. Br J Clin Pharmacol. 2006;61:650-665.
- [CrossRef] [Google Scholar]
- Herbal medicine commonly used against infectious diseases in the tropical island of Mauritius. J Herb Med. 2012;2:113-125.
- [CrossRef] [Google Scholar]
- Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1-93.
- [CrossRef] [PubMed] [Google Scholar]
- Herbal medicines in African traditional medicine In: Builders PF, ed. Herbal Medicine. London, UK: IntechOpen; 2019. p. :191-214.
- [Google Scholar]
- Antidiabetic compounds from medicinal plants traditionally used for the treatment of diabetes in Africa: A review update (2015-2020) South Afr J Bot. 2022;146:585-602.
- [CrossRef] [Google Scholar]
- An appraisal of documented medicinal plants used for the treatment of cancer in Africa over a twenty-year period (1998-2018) J Herb Med. 2020;23:100371.
- [CrossRef] [Google Scholar]
- Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid Based Complement Alternat Med. 2013;2013:617459.
- [CrossRef] [PubMed] [Google Scholar]







