Translate this page into:
Synthesis and anti-tumor activity of piperonal substituted chalcone
*Corresponding author: Zahatu Muhammad, PhD Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria, Nigeria. zahatu2016@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Muhammad Z, Yau J, Zezi AU, et al. Synthesis and anti-tumor activity of piperonal substituted chalcone. Am J Pharmacother Pharm Sci 2023; 011
Abstract
Objectives:
Chalcones have been identified as potential antitumor agents with a novel target, the tubulin. The aim of the study was to synthesize a piperonal substituted chalcone and evaluate its in vivo antitumor activity.
Materials and Methods:
Piperonal substituted chalcone was synthesized using Claisen-Schmidt condensation and characterized using various spectroscopic techniques. The lethal dose (LD50) of the synthesized compound was estimated using OECD-425 guidelines in rats. Antitumor activity of the synthesized compound was evaluated on 1-methyl nitrosourea (MNU)-induced mammary tumor in female Wistar rats. Histological evaluation was used to confirm tumor induction and assess treatment with the synthesized compound. The possible mechanism of action of the synthesized compound was elucidated in silico using molecular docking.
Results:
The compound was synthesized and named C2. C2 was found to be relatively safe with LD50 >2000 mg/kg orally. Moreover, C2 exhibited remarkable antitumor activity, at all the tested doses in a dose dependent manner. Histological evaluation of the MNU-induced mammary tumor rats treated with C2 displayed fewer signs of hyperplasia and small numbers of connective tissue with larger lobules when compared with the untreated group. In silico tubulin-binding interactions revealed that the kinetics of C2 binding to tubulin was like that of colchicine. Comparison of crystal structures of tubulin-C2 and tubulin-colchicine complexes showed that the binding mode of C2 to tubulin was like that of colchicine to tubulin and produced the same conformational changes on the tubulin structure as colchicine.
Conclusion:
The synthesized chalcone demonstrated remarkable antitumor activities in MNU-induced mammary tumors in rats possibly through inhibition of tubulin polymerization.
Keywords
Mammary tumor
Chalcones
Tubulin
Paclitaxel
Claisen-Schmidt
INTRODUCTION
Breast cancer is a malignant tumor that starts in the cells of the breast it occurs almost entirely in women, but men can get it, too.[1] Breast cancer accounts for over one-third of the estimated annual 4.7 million cancer diagnosis in females and the second most common tumor after lung cancer in both sexes. It is also the most common female cancer in both developed and developing countries with 55% of it occurring in the developing countries.[2] The uncontrolled, rapid, and proliferation of cancer cells are one of the most difficult afflictions in the world. Utilization of a single biological molecule or pathway as a target therapy is now one of the main stays in cancer management.[3,4]
Chalcone is a generic term given to compounds bearing the 1, 3-diphenyl-2-propen-1-one in which the third carbon α, β-unsaturated carbonyl system (ketoethylenic group-COCH=CH) is used as an adjunct between two aromatic rings A and B. Chalcones are non-chiral small molecules bearing relative molecular mass in the range of 300–600 g/mol with relatively high lipophilicity (Log P ~5–7). They exist as either trans (E) or cis (Z) isomers [Figure 1].[5]

- (E) and (Z) structures of chalcone.
Chalcones consist of a characteristic framework of 1,3-diaryl-2-propen-1-one and represent an attractive scaffold for the design of novel colchicine site ligands that inhibit tubulin assembly.[6] Due to relative ease in the synthesis of chalcones and the presence of potential skeleton of antiproliferative agent in their structures, they have been subjected to modification to enhance their antitumor potential.[7] Extensive efforts have been done on the introduction of chalcones as effective and inexpensive anticancer. Various derivatives of chalcones including heterocyclic analogues (with N, S and O containing compounds) have been reported to possess anticancer activity. However, there is no clear conclusion as to the substitution pattern of the two chalcone rings for better anticancer activity particularly on inhibition of the tubulin.[8] The simple chemistry of chalcones enables easy, efficient, and convenient synthesis with multiple substitutions. A lot of methods and schemes are available for the synthesis; in each of these methods, the most important part is condensation of two aromatic systems (with nucleophilic and electrophilic groups) to yield the chalcone scaffold. Among all methods, the Claisen-Schmidt condensation [Figure 2] is one of the most common. In this reaction, equimolar quantities of substituted aromatic aldehydes are condensed with substituted aromatic ketones in aqueous alcoholic alkali. The synthesis of chalcones with different substitution patterns on the two aromatic rings further allows to explore many desired potential analogs.[9]
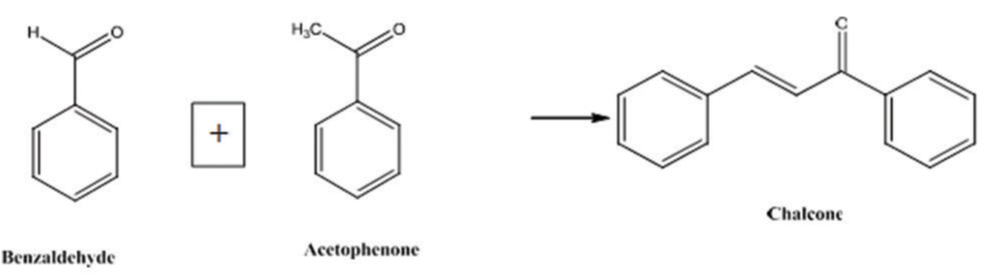
- Claisen-Schmidt condensation.
Microtubules consist of tubulin heterodimers (α and β) which are molecular target of cancer chemotherapy known as microtubule-targeting agents. To overcome the uncontrolled and rapid proliferation of cancer cells, disruption of cellular functions by inhibition of tubulin polymerization or altering the microtubule assembly could be of great importance. Tubulin targeting agents constitute an important class of anticancer drugs and these include taxanes, vinca alkaloids, colchicine, and lot of structurally unrelated small molecules. There are three distinct binding sites on tubulin that are known, namely, the colchicine, taxanes, and the vinca alkaloid binding sites.[10]
Chalcones have toxic effects on cancer cell growth and proliferation. Numerous mechanisms of action have been postulated, such as the inhibition of tubulin assembly, inhibition of angiogenesis, induction of apoptosis, antiestrogenic activity, and reversal of multidrug resistance. Chalcones were reported to possess a good inhibition of tubulin assembly.[11-13] Microtubules function in many essential cellular processes, including mitosis. Tubulin-binding drugs kill cancerous cells by inhibiting microtubule dynamics, which are required for deoxyribonucleic acid segregation and cell division.[14] Molecular docking is an in silico molecular modeling technique that is used to predict how a protein interacts with small molecules. The ability of protein to interact with small molecules to form a supramolecular complex plays a major role in the dynamics of protein which may alter its biological function. This method is aimed at identifying correct poses of ligands in the binding pocket of protein and predict the affinity between the ligand and the protein.[15] The aim of this work was to synthesize and evaluate the antitumor activity of piperonal chalcone.
MATERIALS AND METHODS
Equipment and glassware
Most of the equipment were sourced from the Department of Pharmaceutical and Medicinal Chemistry, Ahmadu Bello University (ABU), Zaria. Some of the equipment used include Magnetic stirrer, stirring bar, refrigerator, gallenklamp melting point apparatus, Analytical balance, microscope, Agilent Fourier Transform Infrared spectrometer, 400 MHz Agilent, and 500 MHz Bruker nuclear magnetic resonance (NMR) spectrometer.
Reagents, solvents, and standard drugs
The reagents were purchased from Sigma Aldrich Germany. All the starting reagents and solvents used for the experiments were of analytical grade and were used without further purification, these include piperonal, acetophenone, sodium hydroxide (50%), iodine crystals, chloroform, ethyl acetate, N-hexane, ethanol, hydrochloric acid, 10% Giemsa stain, acacia, 1-methylnitrosourea (MNU), and paclitaxel.
Synthesis of piperonal substituted chalcone (Claisen-Schmidt condensation)
The chalcone derivative was synthesized through base-catalyzed condensation of 0.1 molar of acetophenone and 0.1 molar of piperonal which were mixed with 20 mL of ethanol in a round bottom flask. To the mixture, 10 mL of 40% sodium hydroxide solution was added drop wise with continuous stirring for 30 min while keeping the mixture cold. The mixing was then continued for another 2 h at room temperature using magnetic stirrer and kept in a refrigerator overnight until it formed a colored solid mass. Drops of concentrated hydrochloric acid were added to neutralize the reaction. The mixture was diluted using 40 mL ice-cold distilled water and then filtered; the residue was washed well with more ice-cold distilled water and dried in air. The product was recrystallized with ethanol, dried, final weight taken, and the percentage yield was calculated. The chemical equations for the synthesis are as shown in [Figure 3]. Silica gel thin layer chromatography was used to monitor the progress of the reaction with ethyl acetate: n-hexane (7:3) as developing solvent.[10] The appearance of a single new spot and disappearance of the reactants spot indicate the formation of the product, which was visualized under 254 nm ultraviolet light and iodine vapor.
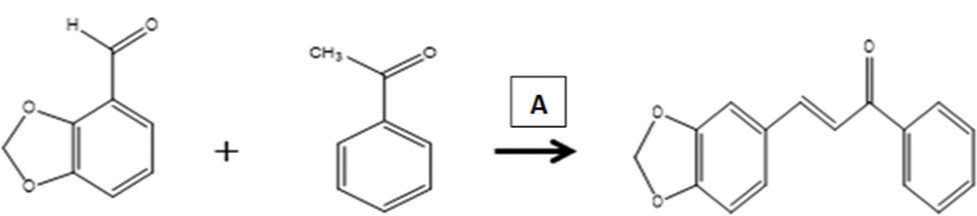
- Scheme for the synthesis of piperonal substituted chalcones Key: A=40% sodium hydroxide.
Characterization of the piperonal substituted chalcone
Melting point was recorded in open capillaries with gallenklamp melting point apparatus. Detailed structural analysis of the synthesized compound was performed using NMR proton (1H) NMR and carbon-13 (13C) NMR.
Data for 1H NMR were reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, dd = doublet of a doublet, m = multiplet), and integration (J in Hz). Data for 13C NMR reported in terms of chemical shift (δ ppm).
Animal management
Ethical approval was sought from the ABU Committee on Animal Use and Care (ABUCAUC) with an approval number of ABUCAUC/2022/003. Sixty female Wistar rats weighing between 60 and 80 g of not more than 50 days of age were obtained and housed in the Animal House of the Department of Pharmacology and Therapeutics, ABU, Zaria. The animals were fed with standard feed and given access to water ad libitum.
Acute toxicity studies in rats
The oral median lethal dose (LD50) was determined using OECD 425 guidelines in rats. Three rats were fasted before treatment for 3 h, fasted body weight was determined for each animal and dose was calculated according to the body weight. Food was further withheld for 2 h after the administration of the new compound. A rat was dosed 2000 mg/kg (limit test) and was observed for 48 h. The first rat survived; thus, additional two rats were dosed 2000 mg/kg. The rats were observed for signs and symptoms of toxicity at least once during the first 30 min, periodically during the first 24 h and then daily for 14 days after dosing.
In vivo anti-mammary tumor study
Female Wistar rats 45–50 days old were used for the study. Mammary tumor was induced according to the method of Thompson et al.[16] with modification. MNU (325 mg) was dissolved in 5 mL of normal saline to form a stock concentration of 65 mg/mL and 65 mg/kg was administered by subcutaneous injection beneath the mammary gland of each rat. The rats were observed and palpated weekly to determine the development, localization, and size of neoplasia on the mammary gland. Eight weeks post-tumor induction, the rats were divided into five groups of six rats each. Rats in Group I served as negative control and received normal saline (1 mL/kg), Groups II, III and IV rats were treated with graded doses of the synthesized compound (50, 25 and 12.5 mg/kg, respectively, through oral route) daily, while rats in Group V served as positive control and received paclitaxel (10 mg/kg, i.p) in alternate days. All the animals were treated for 6 weeks.
Samples collection
At the end of the study, rats were euthanized using chloroform anesthesia, and mammary gland samples were collected, preserved in 10%v/v formalin in normal saline for histological assessment.
Molecular docking
The protein of interest is tubulin and it was obtained from protein data bank with protein data bank (PDB) ID 4O2B. Best resolved monomers were chosen for the study. All nonstandard residues were removed from the 3D structures of the protein using UCSF Chimera. Isolated receptors and cocrystallized ligands were prepared on Chimera and saved as rec.pdb and Lig.mol2, respectively. Rec.pdb and Lig. mol2 were edited on autodock Vina (ADT) by adding polar hydrogen and Gastier charges then saved as pdbqt files.
The ligand studied (Piperonal chalcone) was designed and synthesized at the Department of Pharmaceutical and Medicinal Chemistry, ABU Zaria. The 2D structures were generated using Chem Draw while Spartan was used to convert the 2D structures to 3D. Geometrical optimization using the Austin Model I (AMI) semi-empirical method was performed on the compound using the Spartan software and saved as mol2 file. Hydrogen and Gasteir charges were added on ADT and mlo2 file converted to pdbqt.
Docking procedure for the protein was validated before docking the test compound by separating the cocrystallized ligand from the protein crystal structure and redock using the setup parameters. Procedure that gives conformation superimposable with geometrical conformation of the cocrystallized ligand in the active site was chosen.[17]
Molecular docking was performed considering a flexible ligand and rigid receptor.[18] Docking was carried out using virtual screening software AutoDock Vina.[19] Piperonal substituted chalcone was docked on the active site of the protein. The grid box parameter for the protein is as shown on the [Table 1]. The gridbox parameter was used to write configuration file (config. txt). AutoDock Vina generated results in the pdbqt format. Compounds having the best binding energy, optimal geometric conformation, and broad inhibition of the protein studied were selected from the ViewDock feature of Chimera and saved in complex with the reference protein. The following parameters were accessed using ViewDock; The binding interactions between the compound and the amino acids of the protein, the binding poses of the compound with respect to the protein (active sites) and the conformational changes of the protein with respect to the binding of the compound to the active site.
| X | Y | Z | |
|---|---|---|---|
| Grid box Center | 3.00 | −2.50 | −5.00 |
| Grid box Size | 10 | 10 | 10 |
PDB: Protein data bank
The X, Y, and Z are coordinates that represent the position of the center of the grid box along the three spatial dimensions. The X-axis usually corresponds to the horizontal direction, the Y-axis to the vertical direction, and the Z-axis to the depth direction.
RESULTS
Yield and some physical properties of the synthesized piperonal substituted chalcone
The synthesized compound is a piperonal substituted chalcone with molecular formula of C16H12O3 and was named C2, the molecular formula of chalcone is C15H11O [Table 2], C2 is a yellow powder with a retention factor of 0.44, percentage yield of 95%, and melting point range of 100–102°C. Chalcone is a cream powder with a retention factor of 0.47, percentage yield of 87%, and melting point range of 134.136 [Table 3].
| Compound ID | Molecular formula | 2D-Representation/IUPAC Name |
|---|---|---|
| C2 | C16H12O3 | 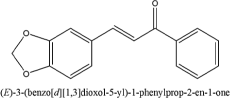 |
| Chalcone | C15H11O |  |
| Compound’s ID | Color | Rfa Values |
Melting points(°C) | % Yield |
|---|---|---|---|---|
| C2 | Yellow powder | 0.44 | 100–102 | 95 |
| Chalcone | Cream powder | 0.47 | 134–136 | 87 |
1Hand 13C NMR of the synthesized chalcone
The 1H and 13C NMR data of the substituted synthesized chalcones are shown in S1 and S2.
C2: 1H-NMR (DMSO) 7.00 (1H, d, J=8.01 Hz), 7.34 (1H, d, J=8.09 Hz), 7.57 (1H, t, J=7.60 Hz), 7.68 (1H, q, J=8.95 Hz), 7.82 (1H, d, J=15.49 Hz), 8.15 (1H, d, J=7.73 Hz).
Acute toxicity study of the synthesized chalcone
The oral median LD50 of C2 was found to be >2000 mg/kg body weight in rats. The preliminary assessment during the first 48 h of the study revealed that, treatment with C2 did not show any critical effects that could lead to death of the rats. Assessment of the rats for 14 days revealed that there was no alteration in functional and behavioral observations following the single administration of C2.
Effect of the synthesized piperonal chalcones on histological examination of MNU-induced mammary tumor in female Wistar rat
Mammary tissues of control rats were shown to have normal fibrous connective tissue, well differentiated ducts, and tiny lobules. The induction of mammary gland tumor, characterized by dilated ducts filled with tumor cells and extreme hyperplasia of mammary lobules and decreased connective tissue, was shown by MNU-induced mammary tumor rats. Rats with MNU-induced mammary tumor treated with C2 displayed fewer signs of hyperplasia and small numbers of connective tissue with larger lobules in a dose dependent way when compared with the untreated group. The group of rats treated with (50 mg/kg) displays the best activity with no signs of hyperplasia, ducts were well-differentiated, and tiny lobules that is almost the same with the control group (plate I).

- Photomicrograph of a section of the mammary gland of female Wistar rats showing the lactiferous glands of the rats treated with normal saline, MNU, and Piperonal substituted chalcone. (a) Normal saline showed a normal feature of the lactiferous gland (H and E ×250), yellow dots = lobules, blue dots = milk ducts, green dots = connective tissues. (b) 1-Methyl nitrosourea (MNU) and normal saline showing dilated ducts filled with tumor cells and extreme hyperplasia of mammary lobules and decreased connective tissue of the lactiferous gland (H and E ×250), yellow dots = lobules, red dots = tumor cells, green dots = connective tissues. (c) 1-MNU and Paclitaxel (10 mg/kg) showing dilated ducts filled with tumor cells and moderate hyperplasia of mammary lobules and slightly decreased connective tissue (H and E × 250), yellow dots = lobules, red dots = tumor cells, green dots = connective tissues. (d) 1-MNU and C2 (12.5 mg/kg) displayed fewer signs of hyperplasia and small numbers of connective tissue with larger lobules (H and E ×250), yellow dots = lobules, red dots = tumor cells, green dots = connective tissues. (e) 1-MNU and Piperonal substituted chalcone (25 mg/kg) displayed fewer signs of hyperplasia and small numbers of connective tissue with larger lobules (H and E ×250), yellow dots = lobules, blue dots = milk ducts, green dots = connective tissues. (f) 1-MNU and Piperonal substituted chalcone (50 mg/ kg) displayed no sign of hyperplasia and normal feature of the lactiferous gland (H and E ×250), yellow dots = lobules, blue dots = milk ducts, green dots = connective tissues.
Molecular docking
The docking procedure applied on the protein was validated. As shown on Plate II, the cocrystallized ligand (ash) and redocked ligand (colored by element) on its respective protein are well super imposed on its original PDB structures.

- Molecular docking validation with the cocrystallized ligand (colchicine) of tubulin protein (PDB ID 4o2b) (a) alpha = alpha-tubulin, beta = beta-tubulin, L = docked Colchicine (colored by elements) binding in 4O2B protein (b) alpha = alpha-tubulin, beta = beta-tubulin, L = docked Colchicine (colored by elements), R = co-crystalized ligand (blue) binding in 4O2B protein.
Docking results indicated that C2 exhibited better binding energies to 4O2B (−9.5 Kcal/mol) than the cocrystallized ligand (−7.5 Kcal/mol). The binding conformation and interaction with amino acid residues on the active site of the protein studied showed formation of bonds between the atoms of C2 and amino acids of 4O2B (Plate III).
![2D intermolecular interactions between docked compounds (Colchicine® and synthesized chalcones [C2]) and 4O2B protein.](/content/127/2023/2/1/img/AJPPS-2-11-g006.png)
- 2D intermolecular interactions between docked compounds (Colchicine® and synthesized chalcones [C2]) and 4O2B protein.
Tubulin-binding interactions revealed that the kinetics of C2 binding to tubulin were like that of colchicine, with evidence of competition between C2 and colchicine for binding site (Plate IV and V). Cocrystal ligand and C2 were well superimposed on the 4O2B protein (Plate VI).

- 3D binding pose of colchicine on its binding cavity, yellow = colchicine.

- 3D binding pose of C2 on its binding cavity, yellow = C2.

- Lid ribbon representation of 4O2B docked with the C2 (colored by element) at the active site and superimposed with colchicine (pink).
Superimposing the crystal structure of the apo-tubulin (unliganded) onto the tubulin-C2 complex reveals that a tubulin loop turn over and curved on the complex while straighten at the apo-tubulin (Plate VII).

- Lid ribbon representation of 4O2B docked with the crystal structure of C2 (green) at the active site (red) and the ApoTubulin (ash). The black circles indicate the structural difference between the two proteins.
DISCUSSION
The synthesis of C2 furnished good yields, this agrees with the synthesis of chalcones and other chalcone derivatives reported in the literatures.[20,21] The sharp melting points observed with the C2 suggests that the compound has high degree of purity.[22]
The 1H NMR and 13C NMR spectra were used to elucidate the structures of the compound. A noticeable change from the starting materials is the disappearance of the aldehyde and methyl ketone chemical shifts, which appear as singlet around 9.89 ppm and 2.50 ppm, respectively. These protons are replaced by the α, β-unsaturated ketone linker protons observed as doublets in the region of 7.5 ppm (Hα) and 8.1 ppm (Hβ) with coupling constants of 15.5 Hz. It is evident from these coupling constants that the product formed is predominantly the trans-isomer (E-form). The configuration of the Z isomer is unstable due to the strong steric effects between the carbonyl group and the A-ring.[22] The rest of the protons appear in their expected regions with their usual coupling constants.
Additional support for the structure of the compound comes from the 13C NMR spectra, where the carbonyl carbon (C=O) of the α, β-unsaturated ketone linker is observed in the region 189.5 ppm, compared to the aldehyde carbonyl group (C=O) at 195 ppm and methyl ketone carbonyl group (C=O) which appears in the region 179 ppm. The α and β-carbon atoms with respect to the carbonyl group give rise to characteristic signals in between δ 120.5 ppm and δ 144.5 ppm, respectively. The olefinic carbons (α-and β-carbon atoms) are evidence that the chalcone have been formed. The positions of all C atoms in the compound have been assigned.
The LD50 of a compound provides an insight on the potential toxicity of that compound and serves a guide of the dose to be used for pharmacological activity of that compound.[23] C2 was found to be relatively safe with LD50 above 2000 mg/kg orally. This provided initial evidence of the potential safety of the compound. Furthermore, lack of mortality and observable adverse effects from C2 in the treated rats throughout the observation period of 14 days further supports the potential safety of C2 following single dose administration.
The antitumor activity of C2 was accessed by the histological assessments of the mammary glands. Histological evaluation is a crucial step in the assessment of breast cancer and helps to determine the type, grade, and stage of the disease, which are important factors in guiding treatment decisions and determining prognosis. Histological features of breast cancer (ductal carcinoma) include inflammation and dilation of duct that is filled with tumor cells, hyperplasia of the lobules, and decrease number of connective tissues.[24] All the features were shown in all the groups of rats that were treated with MNU, hence confirmed the induction of cancer. Treatments with C2 were found to have a remarkable antitumor activity, having activity better than that of paclitaxel (10 mg/kg) at all the tested doses in MNU-induced mammary tumor in rats. The antitumor activity of the compound was more at the highest dose (50 mg/kg p.o), this may imply that the activity of the compound is dose-dependent. Chalcones have been shown to exert cytotoxic activity against many cancer cells lines through multiple mechanisms such as inhibition of angiogenesis, inhibition of cell proliferation and induction of apoptosis.[25] The ability of C2 to inhibit or kill the cancer cells induced by MNU could be suggestive of the activity of C2 through either or all the mechanisms. The antitumor activity seen in the compound is in line with the work of Dong et al.,[7] who reported that synthesized chalcones exhibited an IC50 of <1 µM against K562 cells and the most active compound was impressively cytotoxic and inhibited tubulin polymerization.[7] Another study by Zingales and Moore reported that synthesized chalcone contained potential leading skeletons of antiproliferative agents, and modifications in their skeletal structure enhanced their antitumor activities.[26]
Since the synthesized compound showed promising anticancer activity in vivo, the possible mechanism of the anticancer activity was elucidated using molecular docking. The docking procedures applied on the protein (4O2B) were well validated. From the docking scores, it can be deduced that the compound studied have good affinity for 4O2B and showed appreciable theoretical inhibition of the tubulin. Tubulins are targets for anticancer drugs such as the vinca alkaloids and paclitaxel.[7] Tubulin-binding assays revealed that the kinetics of the C2 binding to tubulin were like that of colchicine, with evidence of competition between the C2 and colchicine for binding site. To compare the tubulin-binding mode of C2 with that of colchicine, the crystal structure of the tubulin – C2 was superimposed onto the tubulin – colchicine structure. The compound (C2) superimposed with colchicine, specifically, the piperonal moiety of C2 contact helix of β-tubulin while the A ring of colchicine contacts the same helix of β-tubulin. This implies that C2 might compete with colchicine for binding site when administered concurrently. Since the antiproliferative mechanism of colchicine has been postulated to be through inhibition of tubulin polymerization by binding to tubulin,[27] any compound that is capable of binding at the colchicine binding site in the tubulin will inhibit the tubulin polymerization and have antiproliferative activity. The ability of the compound to bind on the colchicine binding site could explain the antitumor activity of C2 in MNU-induced mammary tumor in rats. When a compound (ligand) binds to a protein, it forms bond with the amino acids of the protein its bind to thus, result in the alteration of the function of that protein.[28] With respect to binding interaction of C2 with the amino acids of 4O2B, it binds more comfortably to an opposite site of colchicine binding site with a better interaction by forming more hydrogen bonding and lower binding energy than colchicine. This explains the reason of a lower binding energy of C2 when compared to that of colchicine hence the possibility of C2 to have more affinity to 4O2B than colchicine. Formation of hydrogen bond is evidence of good interaction and possibility of the compounds to have high affinity for the binding site; hence, it might address the problem of resistance that is associated with most of the anticancer drugs with anti-tubulin inclusive.[29]
Comparison of the tubulin structure in the bound and unbound states showed that the side chains of the loop residues Leu248 and Ala250 of β-tubulin occlude the colchicine site in the unliganded structure. This observation suggests that, for C2 to bind, the loop must flip outward, and the loop of α-tubulin changes its conformation to accommodate the ligand this results in the destabilization of the microtubules. Similar conformational changes were observed after colchicine binding to tubulin.[30] It was reported that the microtubule-destabilizing activity of colchicine can be explained by the binding of colchicine to the tubulin preventing the curved to straight structural transition in tubulin, a process that is necessary for microtubule formation.[31,32] Together, these data demonstrated that the antitumor activity of C2 could be as a result of their ability to destabilize microtubules by binding to the colchicine binding site and an allosteric site of tubulin.
CONCLUSION
Synthesized chalcone analogs demonstrated remarkable antitumor activities in MNU-induced mammary tumor in rats possibly through inhibition of tubulin polymerization and were found relatively safe in Wistar rats following single administration.
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Conflicts of interest
There are no conflicts of interest.
Supplementary figures available on:
https://doi.org/10.25259/AJPPS_11_2023
Financial support and sponsorship
None.
References
- Surgical management of benign and borderline phyllodes tumors of the breast. Breast J. 2016;22:547-552. doi:10.1111/tbj.12623
- [CrossRef] [PubMed] [Google Scholar]
- Global Cancer Observatory: Cancer Today. 2021. Lyon: International Agency for Research on Cancer; Available from: https://gco.iarc.fr/today [Last accessed on 2023 Mar 15]
- [Google Scholar]
- From single-to multi-target drugs in cancer therapy: When aspecificity becomes an advantage. Curr Med Chem. 2008;15:422-432. doi:10.2174/092986708783503212
- [CrossRef] [PubMed] [Google Scholar]
- Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem. 2014;77:422-487. doi:10.1016/j.ejmech.2014.03.018
- [CrossRef] [PubMed] [Google Scholar]
- Chemical and structural properties of chalcones. FABAD J Pharm Sci. 2011;36:223-242.
- [Google Scholar]
- The compound millepachine and its derivatives inhibit tubulin polymerization by irreversibly binding to the colchicine-binding site in β-Tubulin. J Biol Chem. 2018;293:9461-9472. doi:10.1074/jbc.RA117.001658
- [CrossRef] [PubMed] [Google Scholar]
- Novel natural product-and privileged scaffold-based tubulin inhibitors targeting the colchicine binding site. Molecules. 2016;21:1375. doi:10.3390/molecules21101375
- [CrossRef] [PubMed] [Google Scholar]
- The novel microtubuledestabilizing drug bal27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J Mol Biol. 2014;426:1848-1860. doi:10.1016/j.jmb.2014.02.005
- [CrossRef] [PubMed] [Google Scholar]
- New arylthioindoles: Potent inhibitors of tubulin polymerization. 2. Structure activity relationships and molecular modeling studies. J Med Chem. 2006;49:947-954. doi:10.1021/jm050809s
- [CrossRef] [PubMed] [Google Scholar]
- Reinvestigation of structure-activity relationship of methoxylated chalcones as antimalarials: Synthesis and evaluation of 2,4,5-trimethoxy substituted patterns as lead candidates derived from abundantly available natural β-asarone. Eur J Med Chem. 2010;9:5292-5301. doi:10.1016/j.ejmech.2010.08.049
- [CrossRef] [PubMed] [Google Scholar]
- Inhibitors and promoters of tubulin polymerization: Synthesis and biological evaluation of chalcones and related dienones as potential anticancer agents. Bioorg Med Chem. 2011;19:2659-2665. doi:10.1016/j.bmc.2011.03.005
- [CrossRef] [PubMed] [Google Scholar]
- Combretastin-like chalcones as inhibitors of microtubule polymerization. Part 2: Structure-based discovery of alpha-aryl chalcones. Bioorg Med Chem. 2009;17:7711-7722. doi:10.1016/j.bmc.2009.09.039
- [CrossRef] [PubMed] [Google Scholar]
- Chalcones: A new class of antimitotic agents. J Med Chem. 1990;33:1948-1954. doi:10.1021/jm00169a021
- [CrossRef] [PubMed] [Google Scholar]
- Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9:790-803. doi:10.1038/nrd3253
- [CrossRef] [PubMed] [Google Scholar]
- The RCSB protein data bank: Integrative view of protein, gene and 3D structural information. Nucleic Acids Res 2017:D271-D281. doi:10.1093/nar/gkw1000
- [CrossRef] [Google Scholar]
- Dose-responsive induction of mammary gland carcinomas by the intraperitoneal injection of 1-methyl-1-nitrosourea. Cancer Res. 1992;51:3411-3415.
- [Google Scholar]
- Antimalarial activity of 4-metoxychalcones: Docking studies as falcipain/ plasmepsin inhibitors, ADMET and lipophilic efficiency analysis to identify a putative oral lead candidate. Molecules. 2013;18:15276-15287. doi:10.3390/molecules18121527
- [CrossRef] [PubMed] [Google Scholar]
- Synthesis, biological evaluation, QSAR analysis, and molecular docking of chalcone derivatives for antimalarial activity. Asian Pac J Trop Dis. 2017;7:8-13.
- [CrossRef] [Google Scholar]
- Synthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activities. Eur J Med Chem. 2016;113:34-49. doi:10.1016/j.ejmech.2016.02.041
- [CrossRef] [PubMed] [Google Scholar]
- Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med Res Rev. 2002;28:155-183. doi:10.1002/med.20097
- [CrossRef] [PubMed] [Google Scholar]
- Designs, synthesis and biological evaluation of colchicine derivatives as novel tubulin and histone deacetylase dual inhibitors. Eur J Med Chem. 2015;95:127-135. doi:10.1016/j.ejmech.2015.03.035
- [CrossRef] [PubMed] [Google Scholar]
- In vivo evaluation and in silico prediction of the toxicity of drepano alpha hard capsules. bioRxiv Preprint. 2019;3:54-62.
- [CrossRef] [Google Scholar]
- Malignant tumors of the breast In: DeVita VT, Lawrence TS, Lawrence TS, eds. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology (11th ed). Philadelphia, PA: Lippincott Williams and Wilkins; 2019.
- [Google Scholar]
- Heterocyclic-fused pyrimidines as novel tubulin polymerization inhibitors targeting the colchicine binding site: Structural basis and antitumor efficacy. J Med Chem. 2018;61:1704-1718. doi:10.1021/acs.jmedchem.7b01858
- [CrossRef] [PubMed] [Google Scholar]
- Design and synthesis of (2-(furanyl) vinyl)-1-tetralone chalcones as anticancer agents. Der Pharma Chem. 2016;8:40-47.
- [Google Scholar]
- Promising targets in anticancer drug development: Recent updates. Curr Med Chem. 2017;24:4729-4752. doi:10.2174/0929867324666170331123648
- [CrossRef] [PubMed] [Google Scholar]
- Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science. 2013;339:587-590. doi:10.1126/science.1230582
- [CrossRef] [PubMed] [Google Scholar]
- Methods and applications of structure based pharmacophores in drug discovery. Curr Top Med Chem. 2013;13:1036-1047. doi:10.2174/1568026611313090006
- [CrossRef] [PubMed] [Google Scholar]
- Variations in the colchicine-binding domain provide insight into the structural switch of tubulin. Proc Natl Acad Sci U S A. 2009;106:13775-13779. doi:10.1073/pnas.0904223106
- [CrossRef] [PubMed] [Google Scholar]
- An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res. 2012;29:2943-2971. doi:10.1007/s11095-012-0828-z
- [CrossRef] [PubMed] [Google Scholar]
- The development of chalcones as promising anticancer agents. IDrugs. 2007;10:42-46.
- [Google Scholar]







